
Top 5 Global Rare Disease Companies: An In-Depth Analysis of Roche, Novartis, Johnson & Johnson, AstraZeneca, and Vertex
In recent years, rare disease treatment has rapidly risen from a niche medical issue to one of the most active core areas of global pharmaceutical innovation.
Advances in gene sequencing technology, breakthroughs in cell and gene therapy, the maturity of RNA technology, and policy incentives for orphan drugs in various countries have collectively propelled this market into a phase of rapid development.
As a global pharmaceutical distribution partner focused on innovative drug accessibility, DengYueMed closely tracks regulatory approvals, clinical progress, and real-world demand, offering a frontline perspective on the evolving rare disease landscape.
Based on sales performance, clinical impact, pipeline depth, technological innovation, and global access, Roche, Novartis, Johnson & Johnson, AstraZeneca, and Vertex Pharmaceuticals stand out among the world’s leading rare disease companies.
I. Roche: A Long-Term Leader in Neurological and Hematological Rare Diseases
Roche has long been deeply engaged in neurological and hereditary hematological disorders and remains one of the most consistently innovative rare disease companies worldwide.
The group’s sales reached CHF 61.5 billion in 2025, with core growth mainly coming from innovative products such as Ocrevus and Hemlibra that target diseases with high unmet needs.
✨ The continued strong sales of multiple blockbuster products for chronic rare diseases have enabled Roche to maintain its leading position in long-term disease management.
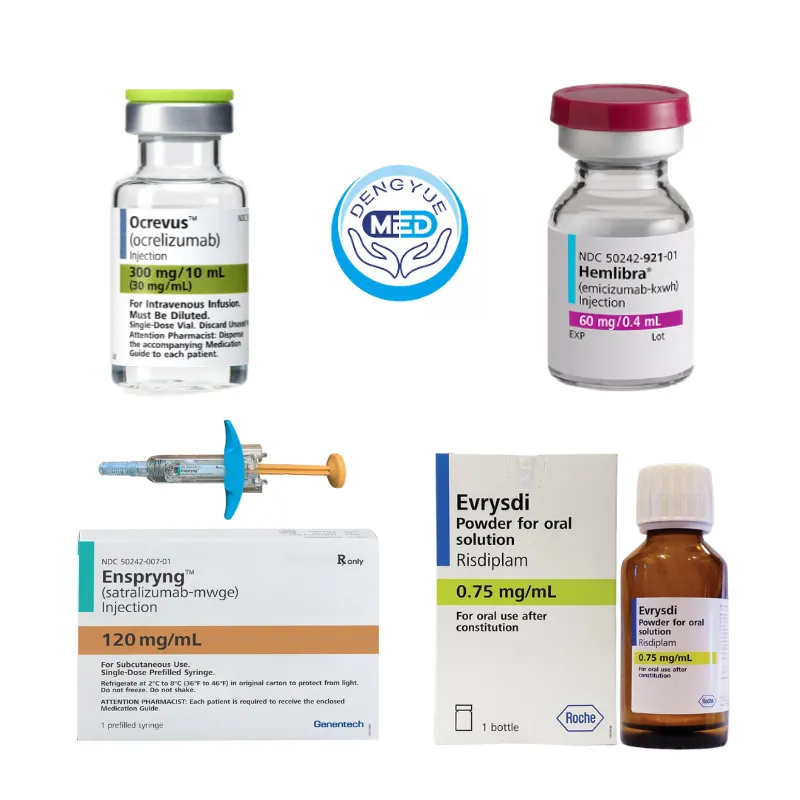
Roche’s Representative Rare Disease Drugs
1️⃣ Ocrevus (Ocrelizumab): Targets CD20-positive B cells, reshaping the long-term disease control model for multiple sclerosis. It is the first major biologic agent to simultaneously cover patients with relapsed and primary progressive multiple sclerosis, and its administration routes are continuously expanding.
2️⃣ Hemlibra (Emicizumab): As one of the most groundbreaking therapies in the field of hemophilia A in the past two decades, Hemlibra uses a bispecific antibody to mimic the function of coagulation factor VIII, significantly reducing the incidence of bleeding and promoting prophylactic treatment as a standard of long-term management.
3️⃣ Evrysdi (Risdiplam): Evrysdi is used to treat spinal muscular atrophy (SMA). It works by regulating SMN2 gene splicing and increasing SMN protein levels, fundamentally slowing disease progression and improving motor function.
4️⃣ Enspryng (Satralizumab): Targeting the IL-6 pathway, it reduces the risk of relapse in neuromyelitis optica spectrum disorders, representing a mature application of precision immune modulation in rare neuroimmunological diseases.
Roche’s Research and Development Pipeline
Roche is continuously advancing multiple clinical studies focusing on the complement system, neurodegenerative diseases, RNA regulation, and precision immune mechanisms.
With a product lifecycle extending to 2030, Roche has identified up to 19 potential new molecular entities, 10 of which are in pivotal clinical stages, forming a core source of growth in rare and severe diseases over the next decade.
II. Novartis: Gene Therapy and a Massive Rare Disease Pipeline
Novartis has long placed rare diseases at the core of its innovation strategy, continuously investing in gene therapy, complement inhibition, and RNA targeting.
Its flagship gene therapy, Zolgensma, has achieved approximately $1.2 billion in annual sales, and the FDA has approved a new dosing regimen for older patients, further solidifying its leadership in SMA.
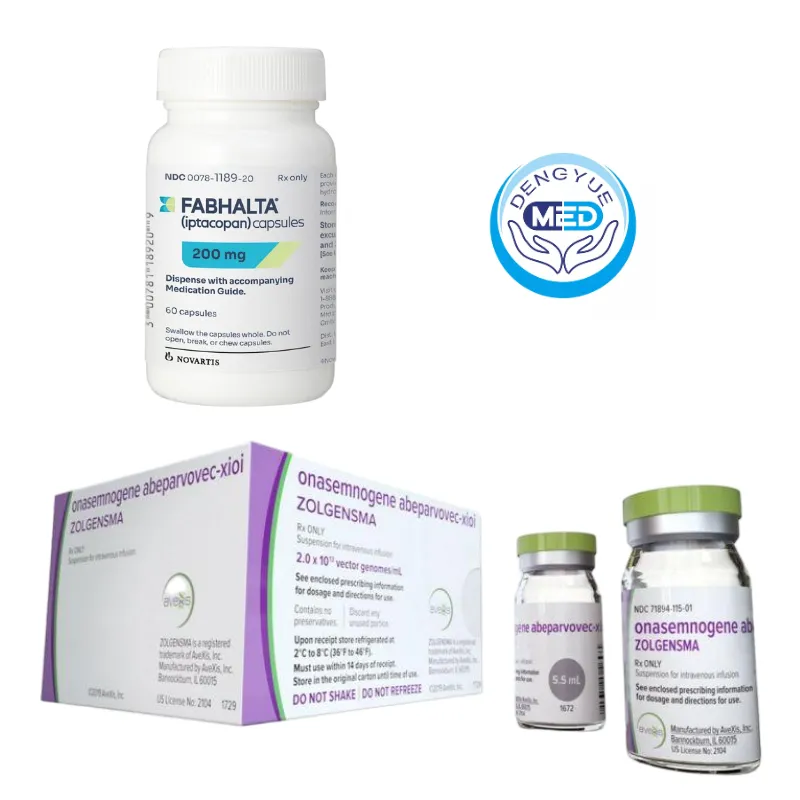
Novartis’ Representative Rare Disease Drugs
1️⃣ Zolgensma (Onasemnogene Abeparvovec): A one-time gene replacement therapy that fundamentally improves neuromuscular function in SMA by supplementing the SMN1 gene, considered a milestone in reshaping the treatment paradigm for rare genetic diseases.
2️⃣ Fabhalta (Iptacopan): Fabhalta, an oral complement factor B inhibitor, has secured key regulatory approvals in multiple complement-mediated rare kidney diseases and is the first approved therapy for C3 glomerulonephropathy, marking the clinical arrival of precise oral complement treatment.
Novartis’ Research and Development Pipeline
Novartis is systematically advancing its diversified innovation strategy:
👉 On one hand, it is strengthening its gene and RNA precision therapy capabilities through strategic collaborations and partnerships with companies such as Avidity Biosciences, which focuses on RNA therapies for hereditary neuromuscular diseases.
👉 On the other hand, the B-cell targeted antibody ianalumab has demonstrated clinical benefits in Phase III studies, reducing disease activity and improving patient burden, and has potential for global application.
III. Johnson & Johnson: Diversified Strategy in Immunology and Hematologic Rare Diseases
With integrated portfolios across multiple myeloma, rare immune disorders, and pulmonary arterial hypertension—supported by CAR-T, bispecific antibodies, and targeted small molecules—Johnson & Johnson stands out among global rare disease companies.
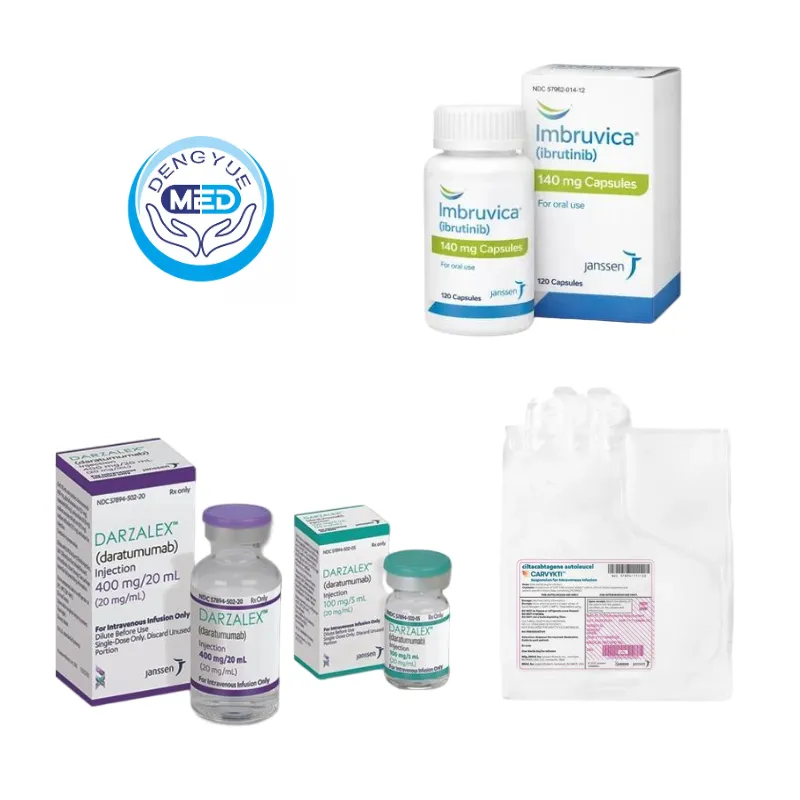
Johnson & Johnson’s Representative Rare Disease Drugs
1️⃣ Darzalex (Daratumumab): As a monoclonal antibody targeting CD38, Darzalex has significantly changed the treatment paradigm for multiple myeloma, a rare hematologic malignancy, and is widely used in multi-line and first-line combination therapies.
It is one of the core cornerstone products of Johnson & Johnson’s hematologic oncology segment.
2️⃣ Carvykti (Ciltacabtagene Autoleucel): BCMA-targeted CAR-T therapy has brought deep and durable remissions to relapsed/refractory myeloma, representing a breakthrough in cell therapy for rare hematologic malignancies.
3️⃣ Imbruvica (Ibrutinib): As a leading BTK inhibitor, Imbruvica has become a long-term standard of care for several rare B-cell malignancies, improving progression-free survival and advancing targeted therapy in rare hematologic diseases.
Johnson & Johnson’s Research and Development Pipeline
Johnson & Johnson is building a systematic rare disease research framework around cell therapy, bispecific antibodies, and FcRn immunomodulation, and is strengthening its innovation sources through acquisitions and collaborations.
In the field of hematologic malignancies, CAR-T combined with bispecific antibody regimens continues to advance, aiming to further improve deep remission rates and prolong progression-free survival.
In the field of immunological rare diseases, FcRn pathway drugs are expanding into various fetal-neonatal and neuroimmunological diseases, forming a cross-disease platform layout.
IV. AstraZeneca: The Rare Disease Engine Centered on Alexion
Since acquiring Alexion, rare diseases have become one of AstraZeneca’s most important growth segments, driven primarily by the continued growth in sales and patient conversion of the long-acting complement inhibitor Ultomiris.
AstraZeneca’s Representative Rare Disease Drugs
1️⃣ Soliris (Eculizumab): As one of the world’s first complement C5 inhibitors, Soliris pioneered the era of targeted therapy for ultra-rare hematologic disorders such as paroxysmal nocturnal hemoglobinuria (PNH) and has long maintained its status as a high-value rare disease drug.
2️⃣ Ultomiris (Ravulizumab): Significantly extending dosing intervals and continuously expanding its application to multiple rare neurological and hematologic diseases has become the core driver of AstraZeneca’s current rare disease revenue growth.
3️⃣ Strensiq, Kanuma, and other metabolic rare disease therapies: Enzyme replacement and targeted therapies from Alexion treat ultra-rare metabolic disorders such as hypophosphatasia and lysosomal acid lipase deficiency, forming a core inherited metabolic disease portfolio for AstraZeneca.

AstraZeneca’s Research and Development Pipeline
AstraZeneca’s rare disease research currently focuses on two core directions: complement pathway and endocrine metabolism.
👉 On the one hand, it continues to deepen the clinical application and long-term efficacy evidence of Ultomiris in various neuroimmunological and hematological rare diseases through lifecycle management.
👉 On the other hand, after completing the integration of Amolyt, it is advancing the late-stage development of the Phase III candidate drug eneboparatide, strengthening its portfolio in endocrine rare diseases such as hypoparathyroidism.
V. Vertex Pharmaceuticals: A Commercial Miracle of Single-Disease Deep Cultivation
Vertex Pharmaceuticals has secured a near-monopolistic global leadership position through its cystic fibrosis drug portfolio, with Trikafta becoming one of the most successful therapies among rare disease companies, generating annual sales approaching $10 billion.
Vertex Pharmaceuticals’ Representative Rare Disease Drugs
1️⃣ Casgevy (Exagamglogene autotemcel): One of the world’s first approved CRISPR gene-editing therapies, it restores normal hemoglobin production by editing hematopoietic stem cells, offering a potential one-time cure for genetic diseases such as transfusion-dependent β-thalassemia, representing a paradigm shift in rare disease treatment.
2️⃣ Trikafta / Kaftrio (Elexacaftor/Tezacaftor/Ivacaftor): This triple CFTR modulation therapy significantly improves lung function and prolongs patient survival, considered a milestone product in cystic fibrosis treatment and a core driver of Vertex’s revenue growth.

Vertex Pharmaceuticals’ Research and Development Pipeline
Vertex Pharmaceuticals is expanding its research focus from cystic fibrosis alone to the fields of kidney, autoimmune, and gene-editing rare diseases.
Among them, the BAFF/APRIL dual-target fusion protein povetacicept is undergoing late-stage clinical trials for IgA nephropathy. Enrollment in the key cohort has been completed, and the company plans to advance its accelerated application in the US in the first half of 2026 based on data progress.
Conclusion: From Breakthrough Innovation to Global Access
Overall, these five rare disease companies represent five different development paths for rare diseases: neurohematologic disorders, gene therapy, immune innovation, complement biology, and single-disease in-depth research.
🎯 Together, they are propelling the global rare disease landscape from “untreatable” to the “era of precision medicine.”
While innovation continues to emerge, how to truly transcend geographical and systemic differences and genuinely benefit more patients with cutting-edge therapies is becoming a new core issue for the industry chain.
As a global pharmaceutical distributor, DengYue Medicine is also playing an increasingly important connecting role in this process—more efficiently translating scientific breakthroughs into real-world treatment opportunities and life improvements.
FAQ about Rare Disease Companies
What is the pharma company for rare diseases?
Several pharmaceutical companies specialize in rare diseases, including Alexion, BioMarin, and Novartis.
What are the top 5 rarest diseases?
There is no official ranking of the rarest diseases, but several affect only a few hundred—or fewer—people worldwide, including fibrodysplasia ossificans progressiva, Fields condition, ribose-5-phosphate isomerase deficiency, Hutchinson-Gilford progeria syndrome, and alkaptonuria.
What is the organization for rare diseases?
Major organizations dedicated to rare diseases include the National Organization for Rare Disorders (NORD), EURORDIS, and Rare Diseases International, which support research, advocacy, and patient communities worldwide.
What are the top 10 biotech companies in the world?
Some of the world’s leading biotech companies include Amgen, Gilead Sciences, Biogen, Moderna, Regeneron, Vertex Pharmaceuticals, BioNTech, Genentech, Illumina, and Seagen.



