Lung Cancer Treatment in 2026: 21 Approved Therapies and the Shift Toward Precision and Immuno-Oncology
Lung cancer has long been one of the leading causes of cancer incidence and mortality worldwide. With rapid advances in molecular biology, immunology, and drug development, lung cancer treatment is undergoing a profound transformation.
In recent years, a wave of innovative targeted therapies and immunotherapies has received regulatory approval, marking a critical shift in lung cancer treatment in 2026—from a historically hard-to-treat disease toward one that can be effectively controlled over the long term, with a subset of patients even achieving clinical remission.
👉 To truly understand how this transformation is reshaping the therapeutic landscape and translating into tangible hope for patients, as explored by DengYue medicine, we must begin with the most fundamental question: what are the major types of lung cancer, and how does precise classification lay the foundation for the “precision strikes” that define modern lung cancer treatment strategies?
Understanding Lung Cancer: Why NSCLC and SCLC Demand Completely Different Treatment Strategies
The classification of lung cancer forms the foundation of all treatment strategies. From a core clinical and decision-making perspective, lung cancer is broadly divided into two fundamental types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC).
This distinction is based not only on the microscopic appearance of cancer cells but more importantly reflects profound differences in disease biology, growth rate, metastatic patterns, and response to therapy—differences that ultimately determine markedly distinct treatment approaches and prognostic trajectories.
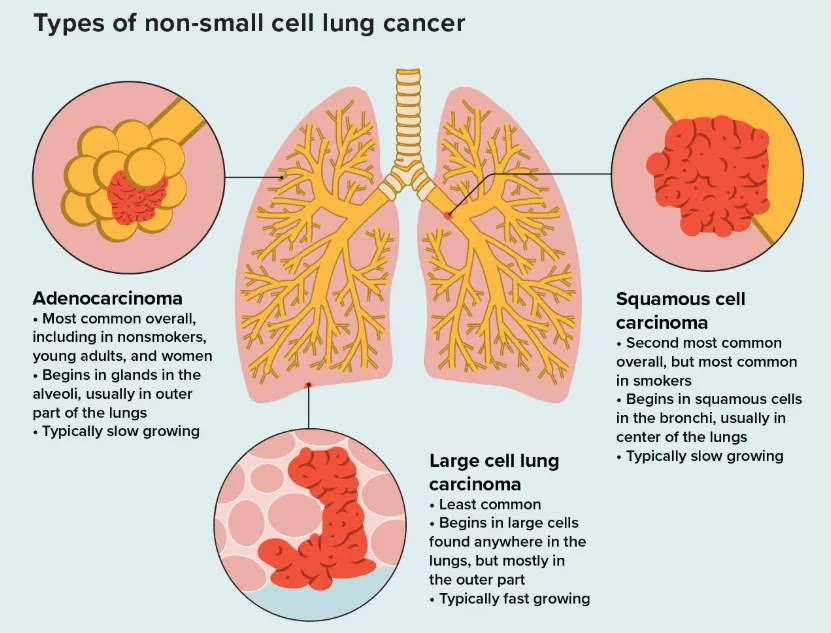
Non-Small Cell Lung Cancer (NSCLC): The Main Battlefield of Precision Medicine
Non-small cell lung cancer is the most common form of lung cancer, accounting for approximately 80%–85% of all cases. Rather than a single disease entity, NSCLC represents a heterogeneous group of tumors, primarily including:
- Lung adenocarcinoma: The most prevalent subtype, particularly common among Asian populations, never-smokers, and women. It is also the subtype with the highest frequency of actionable driver gene alterations.
- Lung squamous cell carcinoma: Strongly associated with smoking and typically arising in the central airways.
- Large cell carcinoma: Relatively rare and generally associated with more aggressive behavior.
Compared with SCLC, NSCLC typically exhibits slower cellular proliferation and clinical progression. Its defining hallmark is pronounced molecular heterogeneity, meaning that tumors from different patients can vary substantially at the genetic and molecular levels.
Through molecular testing, clear oncogenic “driver mutations” can be identified in a significant proportion of patients—particularly those with lung adenocarcinoma. These alterations act as critical molecular switches that sustain tumor growth, including:
- EGFR mutations (present in approximately 40%–50% of Asian patients)
- Gene fusions or rearrangements involving ALK, ROS1, RET, and NTRK
- KRAS G12C mutations
- BRAF V600E mutations
- MET exon 14 skipping mutations and HER2 mutations, among others
The identification of these driver alterations has fundamentally transformed NSCLC treatment—from histology-based chemotherapy to molecularly guided targeted therapy.
This precision-medicine approach significantly improves treatment efficacy, extends survival for patients with advanced disease from months to years, and markedly enhances quality of life.
Small Cell Lung Cancer (SCLC): A Critical Unmet Challenge
Small cell lung cancer accounts for approximately 15%–20% of all lung cancer cases and is characterized by far more aggressive biological behavior.
Small cell lung cancer (SCLC) is characterized by high aggressiveness and early metastatic spread, with an extremely rapid rate of cellular proliferation.
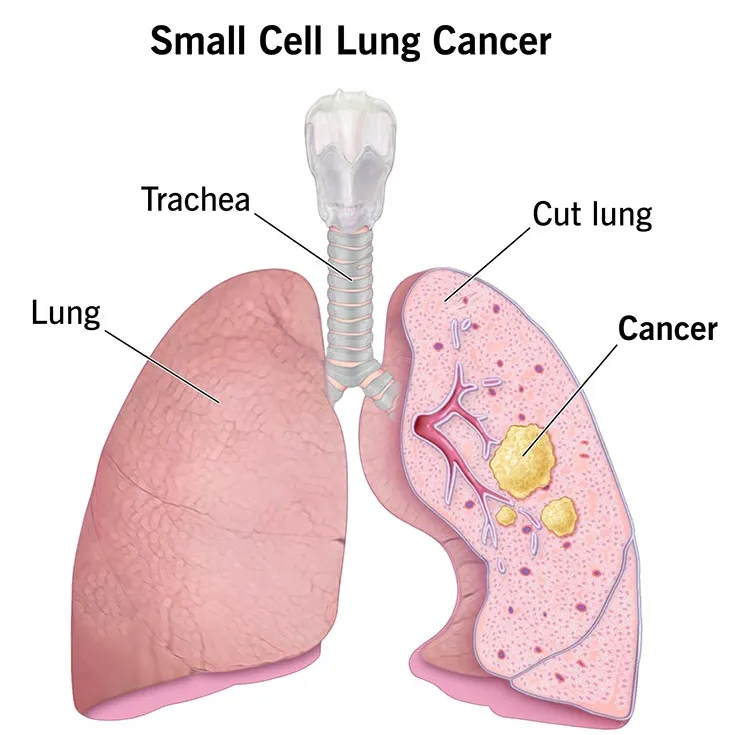
At the time of diagnosis, nearly two-thirds of patients are already in the extensive stage, with metastases to distant organs.
SCLC responds well initially to chemotherapy and radiotherapy, often with rapid tumor shrinkage. However, the vast majority of patients experience early relapse and develop treatment resistance, resulting in persistently poor long-term outcomes. Historically, the five-year survival rate has remained at a very low level.
🤔 For decades, treatment options for SCLC were limited, and effective targeted therapies were lacking.
✅ In recent years, immune checkpoint inhibitors combined with chemotherapy have become the standard first-line treatment for extensive-stage SCLC, providing a clear survival benefit.
✅ In addition, bispecific antibody therapies targeting novel markers such as DLL3 have demonstrated breakthrough activity in clinical trials, opening new avenues for this historically difficult-to-treat disease.
✨ In summary, distinguishing between NSCLC and SCLC is the first critical step toward selecting the right treatment strategy for lung cancer.
NSCLC has led the advance of precision medicine, while SCLC continues to move forward through innovative immunotherapy- and targeted-based combination strategies. Building on this foundation, the next section will explore the key priorities shaping modern lung cancer treatment today.
Modern Lung Cancer Treatment: Key Priorities in the Era of Precision Medicine
With the advent of precision medicine, the treatment philosophy for lung cancer has undergone a fundamental shift—from a “one-size-fits-all” approach to individualized, biology-driven strategies based on tumor classification and molecular characteristics, applied across the entire disease course.
Precision Diagnosis as the Absolute Foundation of Treatment
The core principle of modern lung cancer care is “diagnosis first.” Before any treatment decision is made, a comprehensive and systematic stratification process is essential.
- Pathological classification and clinical staging
1️⃣ Through biopsy, lung cancer must first be classified as NSCLC or SCLC, followed by accurate clinical staging (from stage I to stage IV) using imaging modalities.
2️⃣ This step forms the basis for deciding between curative local treatments and systemic therapies.
- Comprehensive molecular profiling (for NSCLC)
Broad next-generation sequencing (NGS) and PD-L1 expression testing have become standard practice. These tests are not merely used to identify individual targets, but to construct a complete “treatment roadmap”, enabling clinicians to:
- Identify actionable driver alterations, such as EGFR, ALK, ROS1, BRAF, KRAS, MET, RET, HER2, and NTRK, which directly guide targeted therapy selection
- Assess eligibility for immunotherapy, as PD-L1 expression level is a key biomarker predicting the efficacy of immune checkpoint inhibitor monotherapy
- Inform combination strategies, helping determine whether—and how—to combine targeted therapy, immunotherapy, chemotherapy, or anti-angiogenic agents
A Multidimensional, Sequential, and Integrated Treatment Strategy
Guided by precision diagnostics, lung cancer treatment is no longer static. Instead, it is dynamic, combinatorial, and continuous, evolving over the course of the disease.
- Targeted Therapy: The Cornerstone for Driver-Positive Disease
For patients with advanced NSCLC harboring actionable driver mutations, targeted therapies have become the backbone of first-line treatment. By precisely inhibiting oncogenic signaling pathways, these agents have achieved:
- High response rates and prolonged survival: Extending median progression-free survival from 4–6 months with traditional chemotherapy to more than 12 months, with some patients achieving overall survival beyond five years
- Management of resistance through sequential therapy: Particularly for EGFR- and ALK-driven tumors, where multiple generations of targeted agents allow long-term, “chronic disease–like” control
- Immunotherapy: A Central Pillar Reshaping Advanced Lung Cancer Care
The use of PD-1/PD-L1 inhibitors has moved from later lines into the first-line setting and now serves as the backbone of many combination regimens.
- Monotherapy: For patients with high PD-L1 expression (typically ≥50%), immune checkpoint inhibitor monotherapy is a standard first-line option
- Combination strategies:
- Immunotherapy plus chemotherapy is the cornerstone for driver-negative advanced NSCLC
- Immunotherapy combined with anti-angiogenic agents and dual-immunotherapy regimens (e.g., PD-1 plus CTLA-4 inhibition) further expand the population that can benefit
- Expansion into earlier disease stages: Immunotherapy is now used as neoadjuvant or adjuvant treatment for resectable NSCLC, and as consolidation therapy after concurrent chemoradiation for unresectable stage III disease, significantly reducing recurrence risk
- Chemotherapy and Anti-Angiogenic Therapy: Essential Synergistic Components
Despite the rise of targeted and immunotherapies, traditional modalities remain indispensable:
- Chemotherapy: Continues to be the foundation of SCLC treatment and a key partner in combination regimens for driver-negative NSCLC
- Anti-angiogenic agents (such as bevacizumab and anlotinib): Inhibit tumor neovascularization and enhance efficacy when combined with chemotherapy or immunotherapy
Rapid Evolution of Frontier Technologies and Treatment Modalities
The lung cancer therapeutic arsenal is expanding rapidly, bringing new hope to patients with resistant or refractory disease.
- Antibody–drug conjugates (ADCs)
Agents such as datopotamab deruxtecan (TROP2-targeting) and trastuzumab deruxtecan (HER2-targeting) deliver highly potent cytotoxic payloads directly to tumor cells, achieving a new balance between efficacy and safety.
ADCs have become important options for specific molecular subsets, including patients with EGFR-TKI resistance or HER2 alterations.
- Innovative therapeutic approaches under investigation
- Cell-based immunotherapies: Including tumor-infiltrating lymphocyte (TIL) therapy and natural killer (NK) cell therapy, are showing promising activity in clinical trials
- Therapeutic cancer vaccines and mRNA-based technologies: Aim to stimulate or enhance tumor-specific immune responses
- Tumor Treating Fields (TTFields): Alocalized physical therapy using alternating electric fields to disrupt cancer cell division, can be combined with systemic treatments
Longitudinal Management and Supportive Care
Successful modern lung cancer treatment also depends on comprehensive patient management beyond antitumor therapy itself:
- Adverse event management: Proactive prevention and management of toxicities related to targeted therapy, immunotherapy, and chemotherapy are essential to ensure safety and preserve quality of life
- Nutritional and rehabilitative support: Evidence-based nutrition, physical activity, and psychological support improve functional status and treatment tolerance
- Follow-up and monitoring: Regular imaging and molecular surveillance after treatment are critical for assessing response, detecting recurrence or resistance early, and adjusting therapy in a timely manner
In summary, modern lung cancer treatment has evolved into a comprehensive, precision-driven, and patient-centered system—one that begins with accurate diagnosis, integrates targeted therapy, immunotherapy, chemotherapy, anti-angiogenic agents, and emerging technologies, and is sustained by continuous management and supportive care throughout the disease course.
Lung Cancer Therapeutics at a Glance: A Target-Driven Overview of Approved Treatments
Lung cancer has entered a golden era of precision medicine–driven drug development.To date, multiple innovative therapies have been approved globally—particularly in China and the United States—successfully covering eight key therapeutic targets, ranging from common to rare molecular alterations.
These therapies span a broad spectrum of mechanisms, including targeted agents, immune checkpoint inhibitors, antibody–drug conjugates (ADCs), and bispecific antibodies.
To clearly illustrate the full scope of this rapidly expanding therapeutic “arsenal,” the following section summarizes approved treatments by core molecular targets and mechanisms of action.
Targeted Therapies: Precision Inhibition of Oncogenic Drivers
Targeted therapies must be used strictly according to molecular testing results and represent the cornerstone of treatment for driver-positive disease.
- EGFR
- Osimertinib, Aumolertinib, Furmonertinib (third-generation TKIs)
- Sunvozertinib
- First-line treatment for classical EGFR mutations (exon 19 deletion / L858R)
- Sunvozertinib: specifically developed for refractory EGFR exon 20 insertion mutations
- ALK
- Alectinib, Ceritinib, Brigatinib (second-generation TKIs)
- Lorlatinib (third-generation TKI)
- Diroxactib (China approval in 2025)
- Established sequential treatment paradigms for ALK fusion–positive NSCLC
- Diroxactib: approved for treatment-naïve ALK-positive patients
- ROS1
- Crizotinib, Entrectinib
- Taletrectinib (approved in China and the U.S. in 2025)
- For ROS1 fusion–positive NSCLC
- Taletrectinib: demonstrated strong efficacy in ROS1-TKI–naïve patients
- KRAS G12C
- Adagrasib, Sotorasib
- Glecirasib (China approval in 2025)
- Breaks the long-standing “undruggable” barrier
- Glecirasib: provides an important new option for Chinese patients
- MET
- Savolitinib, Capmatinib (oral TKIs)
- Biretinib (China approval in 2025)
- Telisotuzumab vedotin (Teliso-V) (U.S. approval in 2025; ADC)
- For MET exon 14 skipping mutations
- Teliso-V: the first ADC targeting c-MET protein overexpression
- HER2
- Trastuzumab deruxtecan (ADC; foundational agent)
- Zongertinib (approved in China and the U.S. in 2025)
- Sevortinib (U.S. approval in 2025)
- Trastuzumab deruxtecan: reshaped the treatment landscape for HER2-mutant NSCLC
- Novel TKIs provide oral options for pretreated patients
- RET
- Selpercatinib, Pralsetinib
- For RET fusion–positive NSCLC
- Selpercatinib, Pralsetinib
- NTRK
- Larotrectinib, Entrectinib
- Tumor-agnostic therapies for NTRK fusion–positive solid tumors
- Larotrectinib, Entrectinib
Immune Checkpoint Inhibitors: Reactivating Antitumor Immunity
Immune checkpoint inhibitors have become the backbone of first-line therapy for advanced NSCLC and have expanded into perioperative and earlier-stage settings.
| Type | Representative Drugs | Characteristics |
| PD-1 Inhibitors | Pembrolizumab, Nivolumab; Sintilimab, Tislelizumab | 1️⃣ Monotherapy for patients with high PD-L1 expression 2️⃣ Combined with chemotherapy for first-line treatment 3️⃣ Used in neoadjuvant/adjuvant therapy for resectable NSCLC. |
| PD-L1 Inhibitors | Atezolizumab, Durvalumab | 1️⃣ Combined with chemotherapy for NSCLC and extensive-stage SCLC 2️⃣ Consolidation therapy after chemoradiation in unresectable stage III NSCLC |
| Dual Immunotherapy | Nivolumab + Ipilimumab (“O+Y”) | 1️⃣ A chemotherapy-free first-line option for advanced NSCLC with PD-L1 ≥1% and no EGFR/ALK alterations |
Antibody–Drug Conjugates (ADCs) and Bispecific Antibodies
The new generation of “biological missiles,” combining precise targeting with potent cytotoxicity.
- TROP2 ADCs
- Datopotamab deruxtecan
- Sacituzumab govitecan (China approval in 2025)
- For EGFR-mutant NSCLC previously treated with EGFR-TKIs and platinum-based chemotherapy
- Key options in later-line settings
- HER2 ADCs
- Trastuzumab deruxtecan
- Demonstrated outstanding efficacy in HER2-mutant advanced NSCLC
- Trastuzumab deruxtecan
- EGFR/c-MET bispecific antibodies
- Amivantamab (new indication approved in China in 2025)
- For EGFR exon 20 insertion mutations
- In combination with chemotherapy for previously treated EGFR classical mutations
- Amivantamab (new indication approved in China in 2025)
- DLL3 bispecific antibodies
- Tarlatamab (expected China launch in 2026)
- For relapsed or refractory extensive-stage SCLC
- Represents a major breakthrough in this historically difficult-to-treat disease
- Tarlatamab (expected China launch in 2026)
Core Therapeutic Agents for Small Cell Lung Cancer (SCLC)
| Type | Drugs | Notes |
| First-Line Standard Therapy | Chemotherapy (Etoposide/Irinotecan + Platinum) + Immunotherapy (Atezolizumab or Durvalumab) | 🔴 Global standard for extensive-stage SCLC 🟠 Demonstrates significant overall survival benefit |
| Breakthrough New Therapy | Tarlatamab (DLL3-targeting Bispecific T-cell Engager) | 🔴 Brings a paradigm-shifting improvement in later-line treatment 🟠 Already approved by the U.S. FDA |
This continuously expanding list of therapies clearly demonstrates that the future of lung cancer treatment lies in “precise classification and optimal therapy matching.” For patients, comprehensive molecular testing is the essential first step to unlocking access to these advanced treatment options.
As more ADCs, bispecific antibodies, and cell-based therapies move into clinical practice, lung cancer is rapidly transitioning from a fatal disease to a chronic condition that can be effectively managed over the long term.
Lung Cancer
Conclusion
The paradigm shift in lung cancer therapy is redefining the prognosis of this disease at an unprecedented pace. From molecular profiling–based precision classification to targeted inhibition of specific driver genes; from immune checkpoint blockade that unleashes the body’s own defenses to the multidimensional integration of cutting-edge technologies such as antibody–drug conjugates—we are witnessing a new, science-driven era of treatment.
This transformation is not only about extending survival curves, but also about achieving meaningful improvements in quality of life. An increasing number of patients are now able to keep their disease under effective control while maintaining a life of dignity, functionality, and vitality.
In the evolving landscape of Lung Cancer Treatment in 2026, every precise alignment between scientific innovation and individual patient needs lays another foundation of hope—supported by global stakeholders across the treatment ecosystem, including reliable partners such as DengYue, a pharmaceutical wholesaler.
FAQ about Lung Cancer Treatment in 2026
What are the breakthroughs for cancer in 2026?
Prediction: 2026 will mark the year AI moves beyond hype to become an integrated and measurable driver of improved patient care.
What is the future of lung cancer treatment?
Researchers continue to look for new treatment options for all stages of lung cancer. Newer immunotherapies and targeted therapies are showing benefits, and researchers continue to improve these treatments for people with advanced lung cancer.
What is the most successful treatment for lung cancer?
The current standard of care for non-small cell lung cancer includes the use of checkpoint inhibitor immunotherapies, which take the natural “brakes” off the immune system, allowing it to attack cancer cells.
Will 2026 be good for Cancer?
Flaherty sees 2026 as a year where novel chemistry meets earlier care, guided by multimodal precision models that move beyond DNA‑only thinking.