
ALK Inhibitors for Lung Cancer: Generational Evolution and Impact on Clinical Practice
Before the advent of targeted therapy, ALK-positive advanced non-small cell lung cancer (NSCLC) typically progressed rapidly, with a high incidence of brain metastases and limited median survival. Treatment strategies primarily relied on passive control through chemotherapy and radiotherapy.
With the identification of the key driver gene ALK fusion and the continuous emergence of corresponding targeted inhibitors, the natural course of this subtype of lung cancer has been significantly rewritten over the past decade.
From the first to the emerging fourth generation ALK inhibitors for lung cancer, these therapies have progressively improved resistance control and CNS activity while reshaping first-line treatment, brain metastasis management, and long-term survival.
Meanwhile, how to truly bring these innovative therapies into clinical practice in different regions has gradually become a real problem that the global oncology treatment system needs to address.
Based on its global pharmaceutical distribution and supply capabilities, DengYueMed is gradually playing a role in connecting innovation and accessibility in this process.
ALK Inhibitors Mechanism of Action: The Molecular Basis for the Establishment of Targeted Therapy
ALK belongs to the receptor tyrosine kinase family. When the gene undergoes fusion rearrangement, its kinase domain is continuously and abnormally activated, driving multiple pro-tumor signaling pathways, including:
- PI3K-AKT pathway: promoting tumor cell survival
- MAPK-ERK pathway: enhancing cell proliferation
- JAK-STAT pathway: involved in tumor growth and immune escape.
🎯 ALK inhibitors inhibit tumor proliferation and induce apoptosis by competing for ATP binding sites or stabilizing specific kinase conformations, thereby blocking abnormal phosphorylation signal transduction.
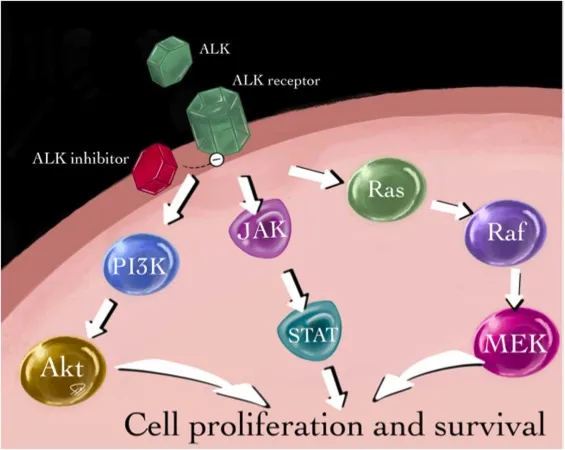
With generational advancements in drug development, their molecular design has gradually evolved from simple signal blocking to broad-spectrum resistance mutation coverage and conformation-selective binding, laying the foundation for sustained clinical benefits.
| Generation | Representative Drugs | Mechanism | Clinical Significance |
| First Generation | Crizotinib | Competitive ATP inhibition | Initially blocks driving signals |
| Second Generation | Ceritinib, Alectinib, Brigatinib | Higher affinity + CNS penetration | Overcomes some drug resistance |
| Third Generation | Loratinib | Stable binding to metacyclic structures | Covers a broad spectrum of mutations |
| Fourth Generation | NVL-655, TPX-0131 | Adapts to complex mutant conformations | Targets deep drug resistance |
First Generation ALK Inhibitors: The Beginning of ALK-Positive Lung Cancer Treatment, Represented by Crizotinib
Crizotinib was the first of the ALK inhibitors for lung cancer to be widely used in patients with ALK-positive disease. Approved by the US FDA in 2011, it transformed the treatment landscape for ALK-positive NSCLC.

Pinnacle randomized studies confirmed that, compared with conventional chemotherapy, ALK-targeted therapy significantly prolonged progression-free survival and improved objective response rates, thus establishing the core position of molecular subtype-driven therapy.
🤔 However, with the widespread clinical use, the limitations of first-generation drugs gradually became apparent: insufficient blood-brain barrier penetration, widespread acquired resistance, and limited duration of efficacy.
These issues directly spurred the development of second-generation ALK inhibitors.
Second Generation ALK Inhibitors: Ceritinib, Alectinib and Other Drugs are Changing the Landscape of First-line Treatment
The core breakthrough of second-generation ALK inhibitors lies in their significantly enhanced CNS penetration and higher inhibitory efficacy.
Clinical studies have shown that these drugs achieve significantly higher intracranial remission rates in measurable brain metastases compared to first-generation treatments, and can extend progression-free survival to the level measured in years.
This evidence directly led to updates in clinical guidelines—highly effective second-generation ALK inhibitors have become the core first-line standard of care for ALK-positive NSCLC. Representative drugs include ceritinib, alectinib, and brigatinib.
More importantly, strong CNS activity reduces dependence on whole-brain or localized radiotherapy, enabling some patients to maintain cognitive function and quality of life for a longer period, marking a shift in treatment goals from simply prolonging survival to maintaining functional survival.
Several Chinese companies are already promoting the production and marketing of generic versions of second-generation ALK inhibitors, such as Aosaikang’s ceritinib capsules, which significantly reduces treatment costs and provides patients with a cost-effective option. DengYueMed supports the supply of such drugs globally.
Third Generation ALK Inhibitors: Lorlatinib Expands Resistance Coverage and Prolongs Disease Control Time
With the widespread use of second-generation drugs, more complex resistance mutations have become a new challenge.
Therefore, lorlatinib, a representative third-generation ALK inhibitor, is designed to:
- Cover a broad spectrum of ALK resistance mutations
- Provide higher CNS exposure levels
- Maintain significant activity after multiple lines of prior therapy

Lorbrena (Lorlatinib) – Lymphoma Kinase | HongKong DengYue Medicine
- Generic Name/Brand Name: Lorlatinib/Lorbrena
- Indications: Lymphoma Kinase
- Dosage Form: tablet
- Specification: 25 mg / 100 mg × 30 tablets
Clinical follow-up results show that a significant proportion of patients achieve disease control for several years, significantly prolonging progression-free survival, and demonstrating outstanding intracranial efficacy in patients with brain metastases.
✨ The emergence of third-generation drugs has also shaken the traditional fixed pattern of “sequential use by generation,” making the use of highly effective ALK inhibitors as first-line treatment a new clinical direction.
4th Generation ALK Inhibitors: NVL-655 and TPX-0131 Explore Deep Resistance Stages
Despite significant progress with third-generation ALK inhibitors for lung cancer, resistance remains a persistent challenge, driving the development of fourth-generation therapies.
These new drugs aim to overcome resistance to lorlatinib, particularly in inhibiting compound mutations.
Fourth-generation ALK inhibitors in early clinical trials (such as NVL-655 and TPX-0131) are attempting to fill the treatment gap following multidrug resistance and may drive two important shifts:
✅ Precision combination therapy becoming the norm: synergistic with other targeted therapies, ADCs, or immunotherapy strategies.
✅ Deepening chronic disease-like long-term management: further extending high-quality survival.
This indicates that the treatment paradigm for ALK-positive lung cancer is shifting from “continuous drug switching” to “long-term control.”
Lung Cancer Treatment Shaped by ALK Inhibitors for Lung Cancer: From Molecular Diagnosis to Long-Term Survival
The generational evolution of ALK inhibitors has had a profound impact on multiple aspects of lung cancer diagnosis and treatment:
1️⃣ Early Diagnosis: Genetic testing has shifted from an optional procedure to a prerequisite for treatment, with NGS widely used in initial diagnosis and progression stages.
2️⃣ Treatment Restructuring: Clinical pathways are gradually taking shape: First-line highly effective targeted therapy → stratified decision-making after drug resistance → multi-line sequential therapy to prolong survival, exhibiting characteristics of chronic disease management.
3️⃣ Improved Quality of Life: High CNS activity therapy reduces the need for radiotherapy, making it more likely that patients can maintain cognitive abilities, social roles, and functional independence in the long term.
Conclusion and Outlook
With the evolution of ALK inhibitors for lung cancer from first-generation therapy to emerging fourth-generation and combination strategies, treatment goals are shifting from short-term remission to durable long-term control and coexistence with disease.
👉 Against this backdrop, pharmaceutical distributors that meet global standards are becoming a key bridge between innovation and clinical application, and HongKong DengYueMed continues to promote the practical accessibility of medicines in this regard.
As continuous drug innovation, precision medicine systems, and global pharmaceutical supply networks gradually synergize, a more manageable and promising era of long-term survival for ALK-positive lung cancer is becoming a reality.
FAQ about ALK Inhibitors for Lung Cancer
What are ALK inhibitors for lung cancer?
ALK inhibitors for lung cancer are targeted therapies across multiple generations—such as crizotinib and lorlatinib—that block abnormal ALK signaling in ALK-positive non-small cell lung cancer.
What medication is used for ALK-positive lung cancer?
ALK-positive lung cancer is primarily treated with targeted ALK inhibitors—most commonly alectinib or brigatinib as first-line therapy, with lorlatinib used for resistant or progressive disease.
What is the FDA approved ALK inhibitor?
Several ALK inhibitors have been approved by the U.S. FDA for ALK-positive non-small cell lung cancer, including crizotinib, ceritinib, alectinib, brigatinib, and lorlatinib, representing multiple generations of targeted therapy.
What are the inhibitors for lung cancer?
Inhibitors used for lung cancer mainly include targeted therapies against specific driver mutations, such as EGFR inhibitors and ALK inhibitors.



