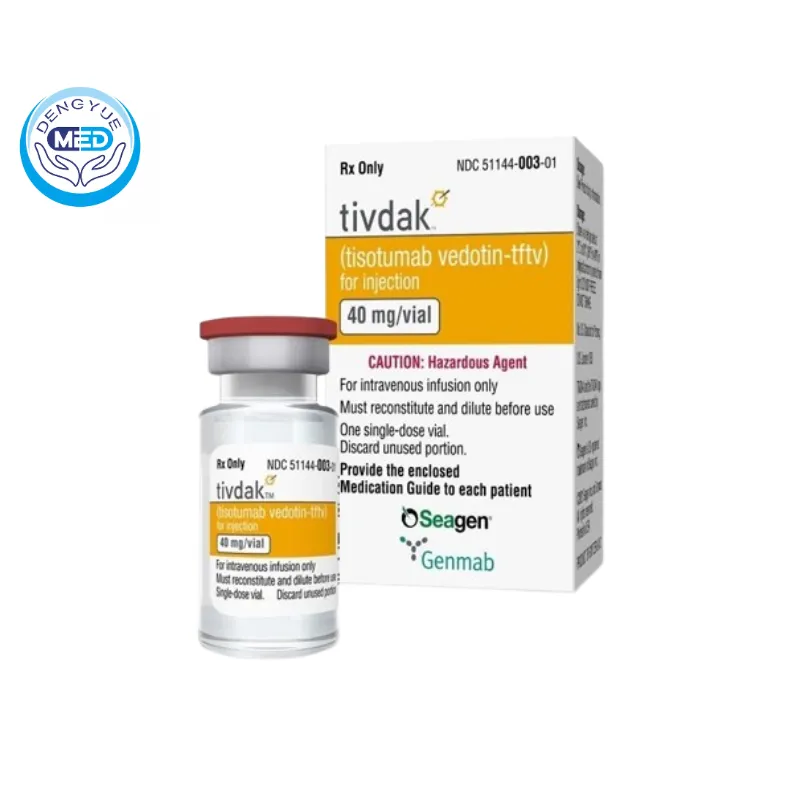
Tivdak (Tisotumab Vedotin) – Uterine Cervical Neoplasms | HongKong DengYue Medicine
- Generic Name/Brand Name: Tisotumab Vedotin/Tivdak
- Indications: Uterine Cervical Neoplasms
- Dosage Form: For injection
- Specification: 40 mg × 1 vial
Tisotumab Vedotin Application Scope
Tisotumab vedotin is indicated for the treatment of adult patients with recurrent or metastatic cervical cancer who have disease progression on or after chemotherapy. It is an antibody–drug conjugate (ADC) targeting tissue factor (TF).
Tisotumab Vedotin Characteristics
-
Ingredients: Tisotumab vedotin-tftv
-
Properties: Lyophilized powder for intravenous infusion after reconstitution.
-
Packaging Specification: 10mL Type I glass vial with grey butyl rubber stopper, plug and top, 20 mm seal with silver coloured aluminium cap and garnet disc. Each carton contains 1 vial containing 40 mg of tisotumab vedotin-tftv.
-
Storage: Store in a refrigerator at 2–8°C.
-
Expiry Date: Varies by manufacturing lot. The dating period for the drug product may be up to 36 months from the date of manufacture when stored at . (Note: The actual expiry date is printed on the package.)
-
Executive Standard: Varies by region
-
Approval Number: Varies by region
-
Date of Revision: 28 March 2025 (EU SmPC). April 2024 (US PI, latest available revision).
-
Manufacturer: Co-developed and co-owned by Genmab and Pfizer (formerly Seagen, which was acquired by Pfizer).
Guidelines for the Use of Tisotumab Vedotin
-
Dosage and Administration:
-
Recommended Dose: 2 mg/kg (up to a maximum of 200 mg) every 3 weeks.
-
Administration: Dilute and administer as an IV infusion over 30 minutes.
-
Missed Dose: If a dose is missed, administer as soon as possible. Adjust the schedule to maintain 3-week intervals.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Alopecia
-
Epistaxis
-
Nausea, vomiting
-
Conjunctival events (dry eye, keratitis, conjunctivitis)
-
Fatigue
-
-
Serious Adverse Reactions:
-
Ocular toxicity (severe corneal ulceration, vision impairment)
-
Peripheral neuropathy
-
Hemorrhage
- Severe infections
-
-
-
Contraindications: Known hypersensitivity to tisotumab vedotin or any component of the formulation.
-
Precautions:
-
Conduct baseline and ongoing ophthalmic examinations.
-
Advise patients to use prophylactic eye drops as recommended.
-
Monitor for bleeding events and peripheral neuropathy.
-
Use effective contraception during treatment and for a period after the last dose.
-
Tisotumab Vedotin Interactions
-
Concomitant use with strong CYP3A4 inhibitors or inducers may alter drug metabolism.
-
Monitor carefully when combined with other agents that may cause ocular toxicity, neuropathy, or bleeding.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.




Reviews
There are no reviews yet.