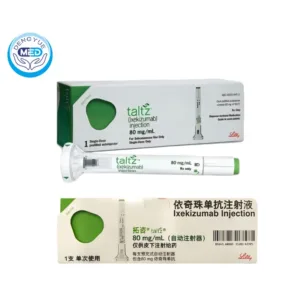
Taltz (Ixekizumab) – Plaque Psoriasis | HongKong DengYue Medicine
- Generic Name/Brand Name: Ixekizumab/Taltz
- Indications: Plaque Psoriasis
- Dosage Form: Injection
- Specification: 80mg/ml x 1 syringe
Taltz Ixekizumab Application Scope
Taltz (ixekizumab) injection is indicated for the treatment of adult patients with moderate to severe plaque psoriasis who are suitable for systemic therapy or phototherapy.
Ixekizumab Injection Characteristics
-
Ingredients: Ixekizumab, a humanized IgG4 monoclonal antibody targeting interleukin-17A (IL-17A).
-
Properties: Sterile, preservative-free, clear and colorless to slightly yellow solution for subcutaneous injection.
-
Packaging Specification: 80mg/ml x 1 syringe
-
Storage: Refrigerate at 2 °C – 8 °C (36 °F – 46 °F), protected from light; do not freeze. May be held at room temperature ≤ 30 °C (86 °F) up to a total of 5 days if needed but then must be used or discarded.
-
Expiry Date: 24 months
-
Executive Standard: JS20240061
-
Approval Number: SJ20190034
-
Date of Revision: See the latest approved package insert.
-
Manufacturer: Eli Lilly and Company
Guidelines for the Use of Taltz
-
Dosage and Administration:
-
Recommended Dose: The recommended dose is 160 mg subcutaneously at week 0 (80 mg twice), followed by 80 mg once at weeks 2, 4, 6, 8, 10 and 12, and then a maintenance dose of 80 mg once every 4 weeks.
-
Administration: Rotate injection sites: abdomen, thigh, upper arm; avoid damaged skin.
-
Missed Dose: If a dose is missed, administer as soon as possible and resume schedule; do not double doses. Refer to official prescribing information for the specific schedule.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Injection site reactions, upper respiratory tract infections, nausea, fungal skin infections (e.g., tinea).
-
Serious Adverse Reactions: Serious infections (bacterial, viral, fungal); opportunistic infections, exacerbations or onset of inflammatory bowel disease, serious hypersensitivity (rare anaphylaxis).
-
-
Contraindications: Known serious hypersensitivity to ixekizumab or any excipients. Active tuberculosis (TB) or untreated latent TB.
-
Precautions:
- Assess for infections including TB before and during therapy.
- Use caution in patients with history of inflammatory bowel disease.
- Avoid live vaccines during treatment.
- Monitor for signs of hypersensitivity.
Ixekizumab Interactions
- No significant pharmacokinetic interactions with other drugs expected, as ixekizumab is a monoclonal antibody. (Standard biologic interaction profile.)
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.



Reviews
There are no reviews yet.