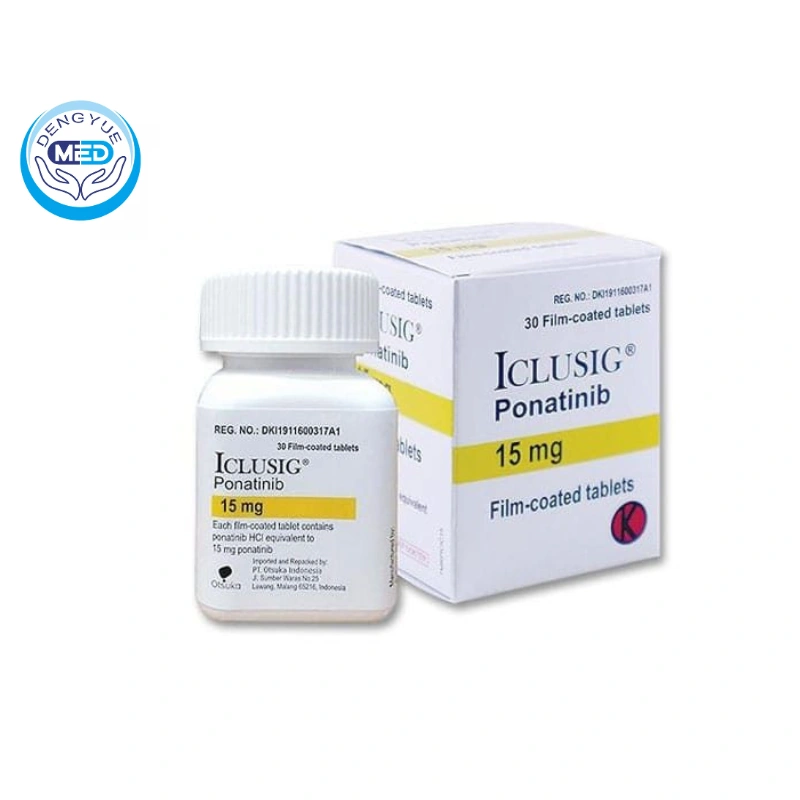
Ponatinib|Acute Lymphoblastic Leukemia|HongKong DengYue Medicine
- Generic Name/Brand Name: Ponatinib/Iclusig
- Indications: Acute Lymphoblastic Leukemia
- Dosage Form: Film-coated tablets
- Specification: 15 mg and 45 mg strengths
Ponatinib Application Scope
Ponatinib, marketed under the brand name Iclusig, is a multi-targeted tyrosine kinase inhibitor primarily used in the treatment of specific types of leukemia. Below is detailed information regarding its application, characteristics, usage guidelines, and interactions.
Ponatinib is indicated for the treatment of adult patients with:
-
Chronic Myeloid Leukemia (CML): Effective in chronic phase (CP), accelerated phase (AP), or blast phase (BP) CML, particularly in patients who are resistant or intolerant to prior tyrosine kinase inhibitor (TKI) therapy, or those with the T315I mutation.
-
Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ ALL): Used in patients who are resistant or intolerant to prior TKI therapy, or those with the T315I mutation.
Ponatinib Characteristics
-
Ingredients: Each tablet contains ponatinib hydrochloride as the active ingredient.
-
Properties: Ponatinib is a potent inhibitor of BCR-ABL1, including forms with the T315I mutation, and other tyrosine kinases.
-
Specification: Available in film-coated tablets of 15 mg and 45 mg strengths.
-
Packaging Specification: Supplied in bottles containing 30 tablets.
-
Storage: Store at room temperature, 20°C to 25°C (68°F to 77°F), with permissible excursions between 15°C and 30°C (59°F and 86°F).
-
Expiry Date: Refer to the packaging for the specific expiration date.
-
Executive Standard: Manufactured in compliance with current Good Manufacturing Practices (cGMP).
-
Approval Number: NDA 203469.
-
Date of Revision: December 2020.
-
Manufacturer: Takeda Pharmaceuticals America, Inc.
Guidelines for the Use of Ponatinib
-
Dosage and Administration:
-
Chronic Phase CML: The recommended starting dosage is 45 mg orally once daily. Upon achieving ≤1% BCR-ABL1IS, reduce the dose to 15 mg once daily. Continue treatment until loss of response or unacceptable toxicity. Consider discontinuation if hematologic response has not occurred by 3 months.
-
Accelerated or Blast Phase CML, Ph+ ALL: The recommended starting dosage is 45 mg orally once daily. Continue treatment until loss of response or unacceptable toxicity. Consider discontinuation if response has not occurred by 3 months.
-
-
Adverse Reactions:
-
Common adverse reactions include hypertension, rash, abdominal pain, fatigue, headache, dry skin, constipation, and arthralgia.
-
Serious adverse reactions encompass arterial occlusive events (e.g., myocardial infarction, stroke), venous thromboembolic events, heart failure, and hepatotoxicity.
-
-
Contraindications:
-
Precautions:
-
Monitor for arterial occlusive events; interrupt and evaluate treatment if such events occur.
-
Assess for venous thromboembolic events; manage as clinically indicated.
-
Regularly evaluate cardiac function; interrupt or discontinue treatment for new or worsening heart failure.
-
Perform liver function tests prior to and during treatment; modify dosage or discontinue for hepatotoxicity.
-
Ponatinib Interactions
-
Drug Interactions:
-
Avoid concomitant use with strong CYP3A inhibitors (e.g., ketoconazole, clarithromycin) as they can increase ponatinib plasma concentrations.
-
Avoid concomitant use with strong CYP3A inducers (e.g., rifampin, phenytoin) as they can decrease ponatinib plasma concentrations.
-
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine. It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.






Reviews
There are no reviews yet.