Pemazyre (Pemigatinib) – Cholangiocarcinoma | HongKong DengYue Medicine
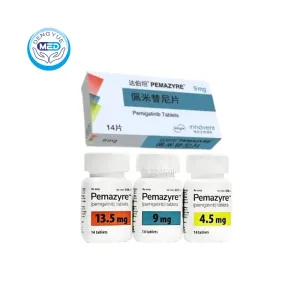
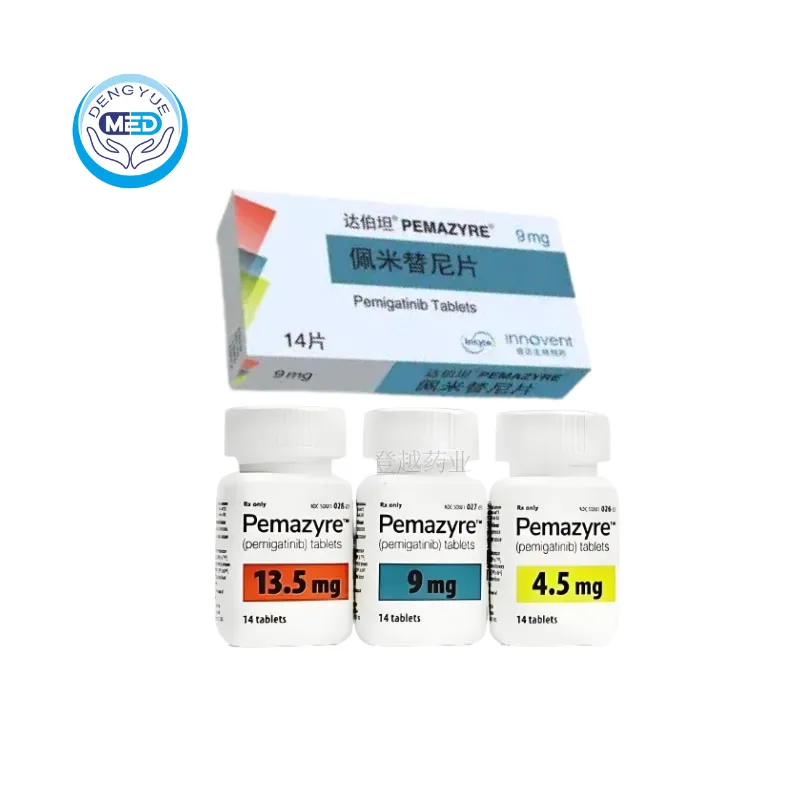
- Generic Name/Brand Name: Pemigatinib / Pemazyre®
- Indications: Cholangiocarcinoma, MLNs
- Dosage Form: Oral tablets
- Specification: 9 mg × 14 tablets/box
Pemigatinib Application Scope
-
Treatment of cholangiocarcinoma with FGFR2 fusions or rearrangements (previously treated, unresectable, locally advanced or metastatic).
-
Treatment of relapsed or refractory myeloid/lymphoid neoplasms (MLNs) with FGFR1 rearrangement.
pemazyre pemigatinib
Pemigatinib Characteristics
-
Ingredients: Pemigatinib
-
Properties: FGFR inhibitor; targeted anticancer therapy
-
Packaging Specification: Film-coated tablets, 4.5 mg / 9 mg / 13.5 mg
-
Storage: Store at 20–25°C (68–77°F); protect from moisture
-
Expiry Date: As per package label
-
Executive Standard: International / FDA / EMA approval standard
-
Approval Number: Varies by region (FDA NDA 212616)
-
Date of Revision: August 2022
-
Manufacturer: Incyte Corporation
Guidelines for the Use of Pemazyre
-
Dosage and Administration:
-
Recommended Dose: 13.5 mg orally once daily, on a 2-weeks-on / 1-week-off cycle
-
Administration: Take orally, with or without food
-
Missed Dose: If missed by ≤4 hours, take as soon as remembered; otherwise, skip and resume next dose
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Hyperphosphatemia
-
Nail toxicity, alopecia
-
Fatigue, diarrhea, dry mouth
-
Stomatitis, dry eyes
-
-
Serious Adverse Reactions:
-
Retinal pigment epithelial detachment (RPED)
-
Severe hyperphosphatemia
-
Hepatotoxicity
-
-
-
Contraindications: Hypersensitivity to Pemazyre or excipients
-
Precautions:
-
Monitor serum phosphate levels regularly
-
Ophthalmologic exams during treatment
-
Adjust dose for severe adverse events or renal/hepatic impairment
-
Pemigatinib Interactions
-
CYP3A4 inhibitors/inducers may alter Pemazyre levels
-
Avoid strong/moderate CYP3A inhibitors (e.g., itraconazole, clarithromycin) and inducers (e.g., rifampin, phenytoin)
-
Dose adjustments may be required with moderate inhibitors
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.




Reviews
There are no reviews yet.