Opdivo (Nivolumab) – NSCLC | HongKong DengYue Medicine
- Generic Name/Brand Name: Nivolumab / Opdivo
- Indications: NSCLC
- Dosage Form: Intravenous injection
- Specification: 100 mg/10ml; 40mg/4ml
Opdivo Application Scope
Opdivo (nivolumab) is a PD-1 immune checkpoint inhibitor that enhances the immune system’s ability to fight cancer by blocking the PD-1 pathway, thereby promoting T-cell activation.
Characteristics
-
Ingredients: Nivolumab
-
Properties: Human IgG4 monoclonal antibody targeting PD-1 receptors on T cells, enhancing immune response against tumors.
-
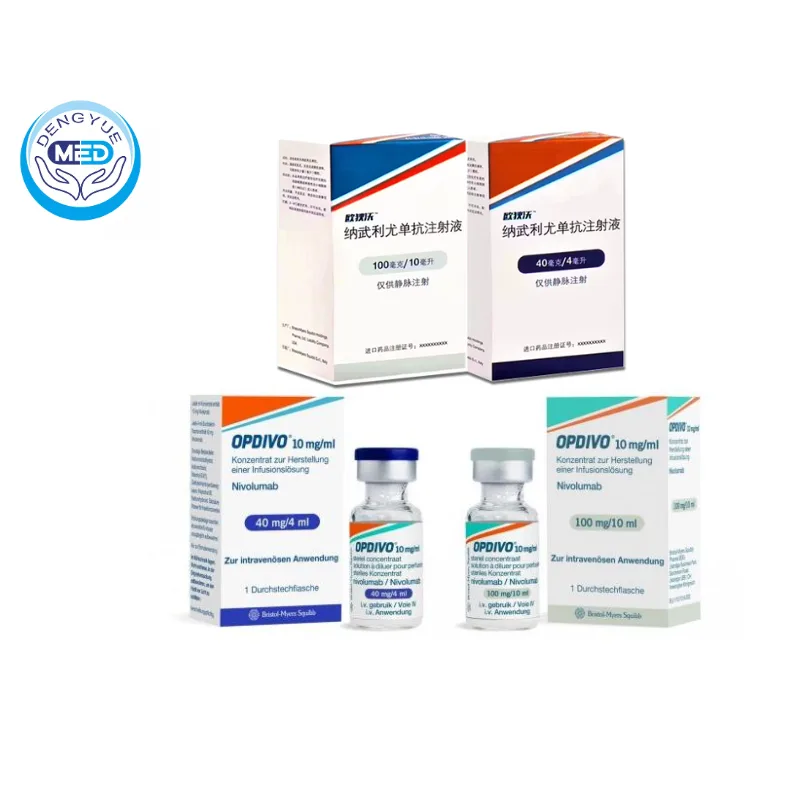
Packaging Specification:
Available in single-dose vials:
-
40 mg/4 mL
-
100 mg/10 mL
-
-
Storage: Store refrigerated at 2°C to 8°C (36°F to 46°F). Do not freeze. Protect from light.
-
Expiry Date: Refer to the specific vial’s labeling for expiration information.
-
Executive Standard: FDA-approved Biologics License Application (BLA) No. 125554.
-
Approval Number: BLA 125554
-
Date of Revision: Refer to the latest prescribing information for the most recent revision date.
-
Manufacturer: Bristol-Myers Squibb Company
Guidelines for the Use of Opdivo
-
Dosage and Administration:
-
Dosage: Dosage varies based on cancer type, stage, and combination with other therapies.
-
Melanoma: 240 mg every 2 weeks or 480 mg every 4 weeks.
-
Non-Small Cell Lung Cancer (NSCLC): 240 mg every 2 weeks or 480 mg every 4 weeks.
-
In combination therapies, dosages are adjusted accordingly.
-
-
Administration: Administered as an intravenous infusion over 30 minutes.
-
-
Adverse Reactions:
-
Common (≥10%):
-
Fatigue, rash, musculoskeletal pain, pruritus, diarrhea, nausea, cough, dyspnea, constipation
-
decreased appetite, back pain, arthralgia, upper respiratory tract infection, pyrexia, headache, abdominal pain, vomiting.
-
-
Serious:
-
Immune-mediated adverse reactions include pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin reactions.
-
-
-
Contraindications:
-
None listed.
-
However, caution is advised in patients with severe hypersensitivity to nivolumab or any of its components.
-
-
Precautions:
-
Monitor for signs of immune-mediated adverse reactions.
-
Use with caution in patients with pre-existing autoimmune conditions.
-
Not recommended during pregnancy; may cause fetal harm.
-
Opdivo Interactions
-
Drug Interactions:
-
No formal drug interaction studies have been conducted.
-
However, immunosuppressive agents may diminish the efficacy of nivolumab.
-
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.








Reviews
There are no reviews yet.