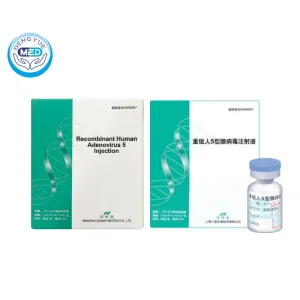
Oncorine (Recombinant Human Adenovirus 5 Injection) – Nasopharyngeal Carcinoma | HongKong DengYue Medicine
- Generic Name/Brand Name: Recombinant Human Adenovirus Type 5 (H101) Injection / Oncorine®
- Indications: Nasopharyngeal Carcinoma
- Dosage Form: Intratumoral injection
- Specification: 5 × 10¹¹ viral particles (VP) x 1 vial
Recombinant Human Adenovirus 5 Injection Application Scope
Oncorine® is a genetically engineered oncolytic adenovirus (H101) approved in China for the treatment of nasopharyngeal carcinoma.
It selectively replicates in tumor cells with p53 pathway defects, leading to tumor regression, particularly when combined with chemotherapy.
Oncorine Recombinant Human Adenovirus 5 Injection Characteristics
-
Ingredients: Recombinant human adenovirus type 5 lacking the E1B 55 kDa gene, enabling selective replication in p53-deficient cancer cells.
-
Properties: Oncolytic viral vector that destroys tumor cells and stimulates anti-tumor immune responses.
-
Packaging Specification: Supplied as a viral suspension; doses vary based on tumor burden and injection protocol (e.g., 0.5×10^12 to 1.5×10^12 viral particles per lesion).
-
Storage: Typically stored frozen; thawed prior to intratumoral administration—standard for viral biologics (details per local labeling).
-
Expiry Date: As per the product label/package insert.
-
Executive Standard: Meets Chinese NMPA biologics and viral therapy specifications, with production quality controls for gene-modified viral agents.
-
Approval Number: Approved by China’s NMPA in November 2005 for nasopharyngeal carcinoma.
-
Date of Revision: Refer to the most recent Chinese package insert or NMPA documentation.
-
Manufacturer: Shanghai Sunway Biotech (H101).
Guidelines for the Use of Recombinant Human Adenovirus 5 Injection
-
Dosage and Administration:
-
Recommended Dose:
-
Administered via intratumoral injection, sometimes repeated daily for several days or every 2–6 weeks, depending on lesion size and treatment regimen.
-
For example, one protocol uses 0.5–1.5×10^12 viral particles per lesion, injected Recombinant Human Adenovirus 5 Injection every 21 days.
-
-
Administration: Delivered directly into the tumor under imaging or endoscopic guidance per clinical protocol.
-
Missed Dose: Typical for multiple-day dosing; dosing reschedules should follow clinical guidelines.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Fever is frequently observed following injection.
-
Serious Adverse Reactions: Generally well tolerated; severe adverse events are rare and usually manageable.
-
-
Contraindications: Patients with active severe infection, immunodeficiency, or uncontrolled systemic illness may not be eligible—needs medical evaluation.
-
Precautions:
-
Monitor for injection-site reactions, systemic inflammatory symptoms, and immune activation.
-
Use in combination with checkpoint inhibitors or chemotherapy under controlled study conditions.
-
Oncorine Recombinant Human Adenovirus 5 Injection Interactions
-
Primarily when combined with other anticancer therapies; no specific pharmacologic drug–drug interactions reported.
-
Investigational combinations include PD-1 inhibitors like nivolumab or tislelizumab, showing promising synergy.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.







Reviews
There are no reviews yet.