Hernexeos (Zongertinib) – NSCLC | HongKong DengYue Medicine
- Generic Name/Brand Name: Zongertinib/Hernexeos
- Indications: NSCLC
- Dosage Form: Film-coated tablets
- Specification: 60 mg × 60
Zongertinib Application Scope
Zongertinib is indicated for the treatment of adult patients with unresectable or metastatic non-squamous non-small cell lung cancer (NSCLC) whose tumors have HER2 (ERBB2) tyrosine kinase domain activating mutations, as detected by an FDA-approved test, and who have received prior systemic therapy. In China, under the trade name Shenghetu, it is approved as monotherapy for adult patients with unresectable, locally advanced or metastatic NSCLC with activating HER2 (ERBB2) mutations who have received at least one line of prior systemic therapy. This indication received conditional approval from China’s NMPA in early September 2025.
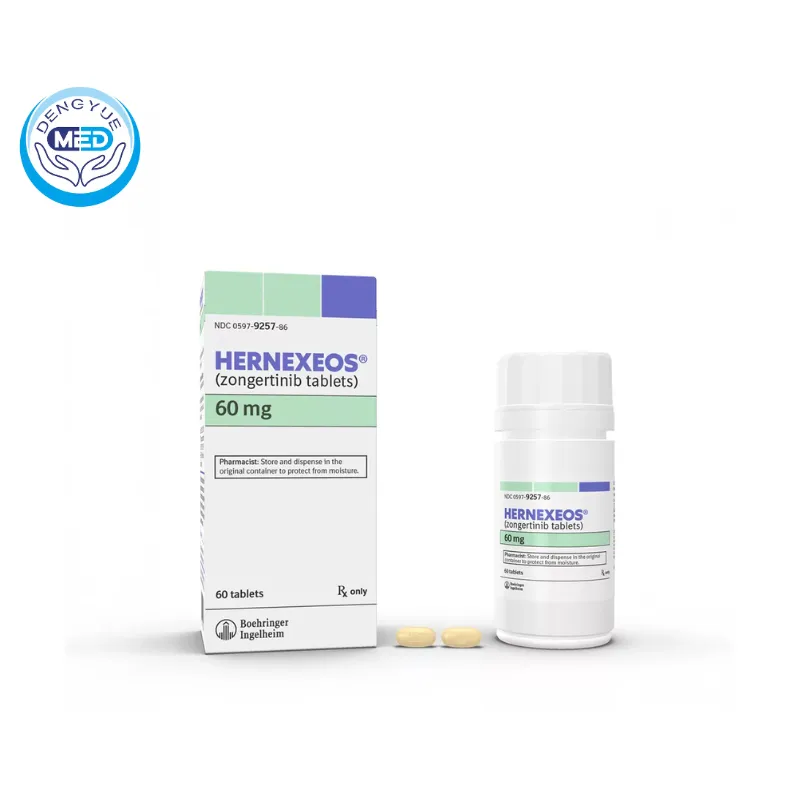
Zongertinib Characteristics
-
Ingredients: Zongertinib (active ingredient).
-
Properties: Yellow, oval, biconvex, film-coated tablets, debossed with “L6” on one side and the Boehringer Ingelheim company symbol on the other side. Each tablet contains 60 mg of zongertinib.
-
Packaging Specification: Bottles of 30 tablets (NDC 0597-0480-30).
-
Storage: Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F). Dispense in the original bottle.
-
Expiry Date: Discard any unused tablets 3 months after opening the bottle.
-
Executive Standard: Aligns with FDA prescribing information standards for the US and NMPA conditional approval standards for China.
-
Approval Number: US FDA approval: August 8, 2025 (NDA 219042). China NMPA conditional approval: Early September 2025
-
Date of Revision: 08/2025 (US prescribing information).
-
Manufacturer: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT 06877 USA (US); Boehringer Ingelheim International GmbH (China development).
Guidelines for the Use of Zongertinib
-
Dosage and Administration:
-
Recommended Dose: Based on body weight: <90 kg: 120 mg once daily; ≥90 kg: 180 mg once daily. Take with or without food.
-
Administration: Swallow tablets whole with water; do not split, crush, or chew. If vomited, do not take an additional dose; resume at the next scheduled time.
-
Missed Dose: If missed by less than 12 hours, take the dose; if more than 12 hours, skip and take the next scheduled dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Diarrhea, rash, decreased appetite, nausea, stomatitis, paronychia, fatigue.
-
Serious Adverse Reactions: Interstitial lung disease (ILD)/pneumonitis, QT prolongation, hepatotoxicity.
-
-
Contraindications:None.
-
Precautions:
-
Monitor for hepatotoxicity (liver function tests at baseline, every 2 weeks for first 3 months, then monthly or as needed); left ventricular ejection fraction (at baseline and during treatment); signs/symptoms of ILD/pneumonitis; embryo-fetal toxicity (advise contraception during and for 7 months after last dose for females, 4 months for males). Avoid breastfeeding during treatment and for 2 weeks after last dose. No dose adjustment for mild/moderate renal or hepatic impairment; not studied in severe impairment.
-
Zongertinib Interactions
-
Avoid concomitant use with strong CYP3A inducers (may decrease zongertinib exposure and reduce effectiveness); if unavoidable, increase dose to 240 mg (<90 kg) or 360 mg (≥90 kg) during coadministration, then resume original dose 7-14 days after discontinuation of inducer. Avoid certain BCRP substrates where minimal concentration changes may lead to serious adverse reactions; if unavoidable, monitor and follow substrate labeling recommendations. Zongertinib is a CYP3A substrate.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.








Reviews
There are no reviews yet.