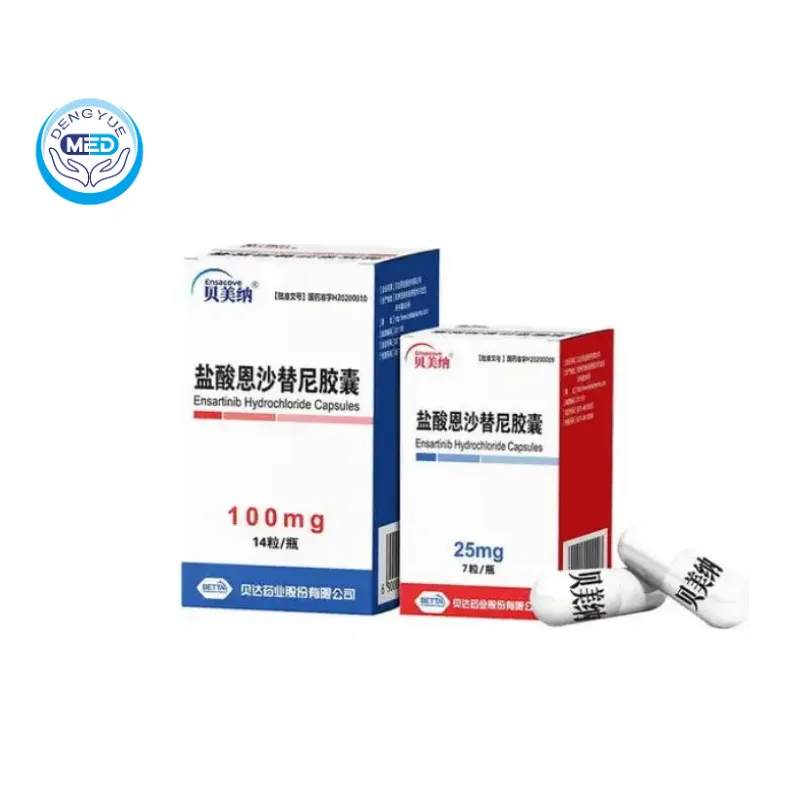
Ensacove (Ensartinib Hydrochloride) – NSCLC | DengYueMed
- Generic Name/Brand Name: Ensartinib Hydrochloride/Ensacove
- Indications: NSCLC (Lung Cancer)
- Dosage Form: Oral capsules
- Specification: 25 mg/100 mg
Ensacove Application Scope
Ensacove is an oral, next‑generation ALK tyrosine kinase inhibitor to treat ALK‑positive non–small cell lung cancer (NSCLC).

Characteristics
-
Ingredients: Ensartinib Hydrochloride
-
Properties:
-
Oral ALK inhibitor with activity against ALK rearrangements, ROS1
-
Designed to penetrate the blood–brain barrier
-
Overcome resistance to earlier ALK inhibitors
-
-
Packaging Specification:
-
Capsules available in 25 mg and 100 mg strengths
-
Supplied in bottles or cartons, NDC-labeled for the U.S. market
-
-
Storage:
-
Store at room temperature, protect from moisture
-
Keep in the original container
-
-
Expiry Date: Provided on the package; follow label-dated expiration guidance
-
Executive Standard:
-
In compliance with FDA (U.S.) and NMPA (China) regulations
-
Per capsule code ENSACOVE/ensartinib
-
-
Approval Number:
-
FDA approved December 18, 2024
-
NMPA China 2020 (second‑line) and 2022 (first‑line) approvals
-
-
Date of Revision: Last label update December 2024
-
Manufacturer:
-
Developed by Xcovery Holdings
-
Distributed in China by Betta Pharmaceuticals
-
Guidelines for the Use of Ensacove
-
Dosage and Administration:
-
Recommended dose:
-
225 mg orally once daily, with or without food
-
-
Continue until disease progression or unacceptable toxicity
-
Capsules should be swallowed whole
-
If a dose is missed, take it within 12 h; don’t double-dose
-
-
Adverse Reactions:
-
Common (≥20%):
-
rash, musculoskeletal pain, constipation, cough
-
pruritus, nausea, edema, vomiting, fatigue, fever
-
-
Lab abnormalities:
-
hyperglycemia, elevated liver enzymes
-
increased CPK, uric acid, electrolyte changes
-
-
-
Contraindications: Known hypersensitivity to ensartinib or capsule excipients
-
Precautions:
-
Monitor for dermatologic reactions (DRESS, photosensitivity)
-
Withhold or reduce the dose based on severity
-
Watch for bradycardia, hyperglycemia, and visual changes
-
Elevated CPK, hyperuricemia—adjust dose or suspend as needed
-
May cause fetal harm—advise contraception
-
Avoid use in patients with severe liver impairment
-
Ensacove Interactions
-
Drug Interactions:
-
Substrate of CYP3A4 and P-gp: avoid co-administration with strong
-
Moderate inhibitors or inducers to prevent altered drug levels and toxicity
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.







Reviews
There are no reviews yet.