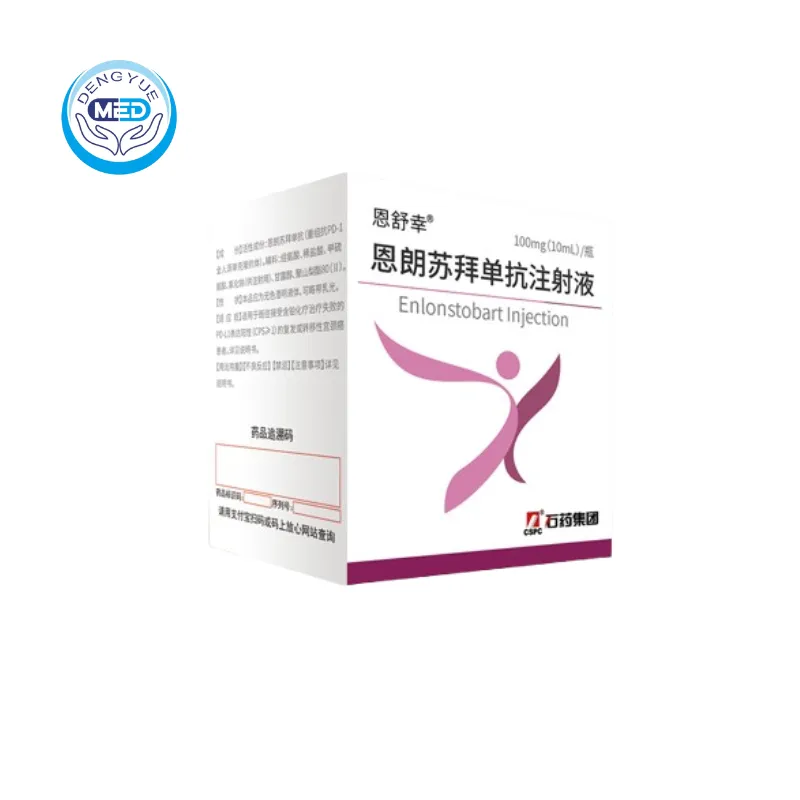
Enshuxing (Enlonstobart) – Cervical Cancer|HongKong DengYue Medicine
- Generic Name/Brand Name: Enlonstobart Injection / Enshuxing®
- Indications: Cervical Cancer
- Dosage Form: injection
- Specification: 100mg × 1 vial/box
Enlonstobart Injection Application Scope
Enlonstobart Injection, marketed under the brand name Enshu Xing®, is a fully human anti-PD-1 monoclonal antibody developed by CSPC Pharmaceutical Group. It received approval from the National Medical Products Administration (NMPA) on June 25, 2024, for the treatment of patients with PD-L1 positive (CPS≥1) recurrent or metastatic cervical cancer who have failed prior platinum-based chemotherapy.

Enlonstobart Injection Characteristics
- Ingredients: Enlonstobart Injection contains a fully human IgG4 subtype anti-PD-1 monoclonal antibody.
- Properties: As a PD-1 inhibitor, it blocks the interaction between the PD-1 receptor on T-cells and its ligands, PD-L1 and PD-L2, thereby enhancing the immune system’s ability to attack tumor cells.
- Specification: Each vial contains 100 mg of the monoclonal antibody in 10 ml solution.
- Packaging Specification: Provided in single-use vials containing 100 mg/10 ml of the solution.
- Storage: Store refrigerated at 2°C to 8°C (36°F to 46°F). Do not freeze.
- Expiry Date: Refer to the product labeling for specific expiration information.
- Executive Standard: Manufactured in compliance with current Good Manufacturing Practices (cGMP) and relevant regulatory standards for biopharmaceutical products.
- Approval Number: S20240028.
- Date of Revision: Refer to the product’s prescribing information for the most recent revision date.
- Manufacturer: CSPC Pharmaceutical Group.
Guidelines for the Use of Enshuxing
- Dosage and Administration: The recommended dosage should be determined by a healthcare professional based on the patient’s condition. Administration is typically via intravenous infusion.
- Adverse Reactions: Common side effects may include fatigue, rash, pruritus, diarrhea, and immune-related adverse events. Notably, as a fully human antibody, Enlonstobart Injection has a lower incidence of immune-related adverse events compared to other PD-1 inhibitors, such as reactive cutaneous capillary endothelial proliferation.
- Contraindications: Contraindicated in patients with known hypersensitivity to Enlonstobart Injection or any of its components.
- Precautions: Use with caution in patients with autoimmune diseases, a history of severe immune-related adverse reactions, or those receiving immunosuppressive therapy. Regular monitoring of liver and thyroid function tests is recommended during treatment.
Enlonstobart Injection Interactions
- Drug Interactions: No significant drug interactions have been reported. However, concurrent use with other immunomodulatory agents should be approached with caution.
For personalized medical advice and detailed prescribing information, it is essential to consult healthcare professionals before initiating therapy with Enlonstobart Injection.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.
Be the first to review “Enshuxing (Enlonstobart) – Cervical Cancer|HongKong DengYue Medicine”Cancel Reply
Contact Us
Related products
-

Tivdak (Tisotumab Vedotin) – Uterine Cervical Neoplasms | HongKong DengYue Medicine
-

Hycamtin (Topotecan Hydrochloride) – Malignancies | HongKong DengYue Medicine
-

Qibeian (Iparomlimab Tuvorlimab) – Cervical Cancer | HongKong DengYue Medicine
-

Sorrento (Socazolimab) – Cervical Cancer | HongKong DengYue Medicine
-

Avastin (Bevacizumab) – mCRC & NSCLC | HongKong DengYue Medicine
-

Aibite (Famitinib Malate Capsules) – Cervical Cancer | HongKong DengYue Medicine
-

Iparomlimab And Tuvonralimab Injection|Cervical Cancer





Reviews
There are no reviews yet.