Emgality (Galcanezumab) – Anti-Migraine | HongKong DengYue Medicine
- Generic Name/Brand Name: Galcanezumab/Emgality
- Indications: Anti-migraine medication
- Dosage Form: Solution for Injection
- Specification: 120 mg × 2 vial
Galcanezumab Application Scope
Galcanezumab is indicated for the prevention of migraine in adults and for the treatment of episodic cluster headache. It helps reduce the frequency of migraine attacks and cluster headache periods by targeting the calcitonin gene-related peptide (CGRP), a key molecule involved in migraine pathophysiology.
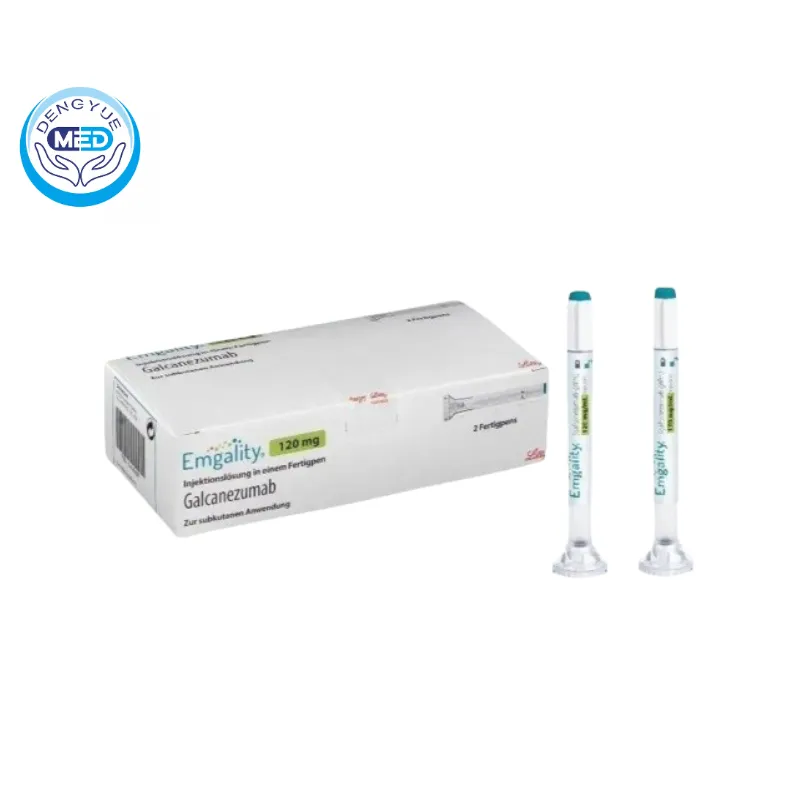
Galcanezumab Characteristics
-
Ingredients: Galcanezumab (as galcanezumab-gnlm)
-
Properties: Galcanezumab is a humanized monoclonal antibody that selectively binds to CGRP, preventing it from binding to its receptor, thereby reducing migraine-related neurogenic inflammation and vasodilation.
-
Packaging Specification: Pre-filled syringe or autoinjector containing 120 mg/mL solution for subcutaneous injection. Each pack typically contains 1 or 2 syringes/autoinjectors.
-
Storage: Store refrigerated at 2–8°C. Do not freeze. Protect from light.
-
Expiry Date: As indicated on the product label.
-
Executive Standard: Complies with the applicable national pharmacopoeia and manufacturer’s internal quality standards.
-
Approval Number: In China: 国药准字SJ20240003. Register certificate number: 2024S00011. In the US: Initial U.S. Approval 2018 (US License Number 1891). In the EU: First authorized 14 November 2018 (renewed 01 September 2023).
-
Date of Revision: Varies depending on the regulatory agency’s latest update (refer to the current official product label).
-
Manufacturer: Lilly Suzhou Pharmaceutical Co., Ltd.
Guidelines for the Use of Galcanezumab
-
Dosage and Administration:
-
Recommended Dose:
For migraine prevention:
-
Recommended dose: 240 mg (two consecutive 120 mg injections) as an initial loading dose, followed by 120 mg once monthly.
For episodic cluster headache:
-
Recommended dose: 300 mg (three consecutive 100 mg injections) at the onset of the cluster period, then every month until the end of the cluster period.
-
-
Administration: Administer by subcutaneous injection in the abdomen, thigh, upper arm, or buttocks.
Rotate injection sites with each dose. -
Missed Dose: If a dose is missed, administer it as soon as possible. Thereafter, resume the regular monthly schedule from the date of the last injection.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Injection site pain or redness
-
Constipation
-
Pruritus
-
Hypersensitivity reactions (mild to moderate)
-
-
Serious Adverse Reactions: Severe hypersensitivity, including angioedema or anaphylaxis (rare)
-
-
Contraindications:
-
Known hypersensitivity to galcanezumab or any excipients.
-
Use in children and pregnant or lactating women has not been fully established; use only if clearly needed and directed by a physician.
-
-
Precautions:
-
Evaluate patients for other causes of headache before initiating treatment.
-
Monitor for signs of hypersensitivity after administration.
-
Discontinue treatment if serious allergic reaction occurs.
-
Safety in severe hepatic or renal impairment has not been fully studied.
-
Galcanezumab Interactions
- No clinically significant drug–drug interactions have been reported.
Galcanezumab does not interact with cytochrome P450 enzymes and is not expected to affect the metabolism of other drugs.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.



Reviews
There are no reviews yet.