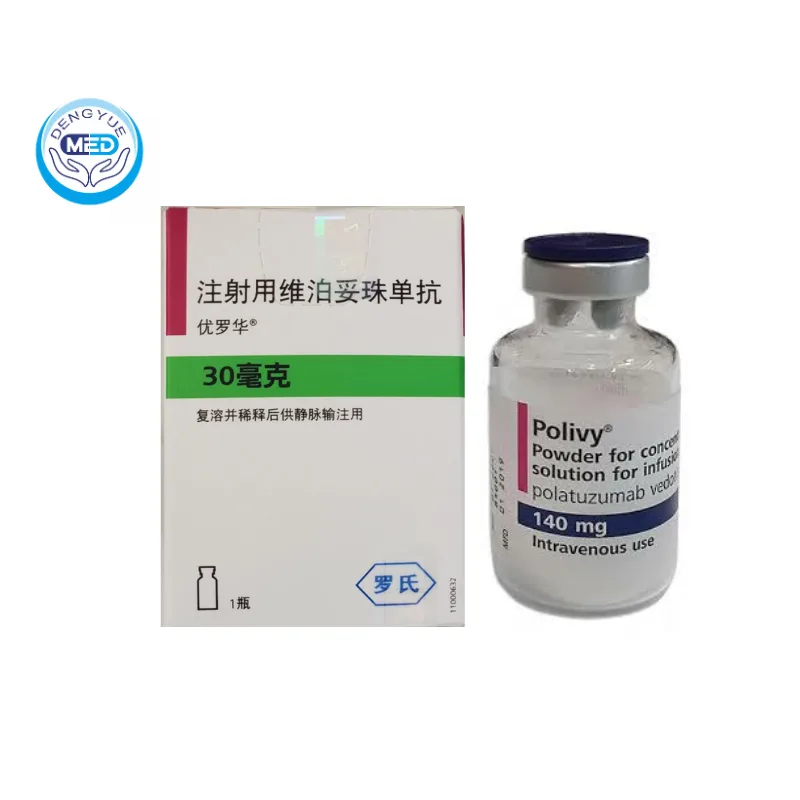
Polivy (Polatuzumab Vedotin) – DLBCL | HongKong DengYue Medicine
- Generic Name/Brand Name: Polatuzumab Vedotin/Polivy
- Indications: Relapsed or Refractory (R/R) DLBCL
- Dosage Form:
- Specification: 30 mg or 140 mg × 1 vial
Polatuzumab Vedotin Application Scope
Polatuzumab vedotin (Polivy) is indicated in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) for the treatment of adult patients with previously untreated diffuse large B-cell lymphoma (DLBCL). It is also indicated in combination with bendamustine and rituximab for the treatment of adult patients with relapsed/refractory DLBCL who are not candidates for hematopoietic stem cell transplant.

Polatuzumab Vedotin Characteristics
-
Ingredients: Polatuzumab Vedotin-piiq
-
Properties: White to off-white lyophilized powder for intravenous infusion after reconstitution.
-
Packaging Specification: Vial containing 30 mg or 140 mg of Polatuzumab Vedotin-piiq.
-
Storage: Store at 2°C–8°C (36°F–46°F); do not freeze. Protect from light.
-
Expiry Date: Refer to the package label.
-
Executive Standard: As per manufacturer’s quality standard or local pharmacopoeia.
-
Approval Number: The dating period for the drug product is typically 24 months from the date of manufacture when stored at 2-8 °C. Always refer to the expiration date printed on the actual drug packaging.
-
Date of Revision: Not available in general public information (consult current prescribing information).
-
Manufacturer: Hoffmann-La Roche Limited / Genentech (depending on the region/country).
Guidelines for the Use of Polatuzumab Vedotin
-
Dosage and Administration:
-
Recommended Dose: The recommended dose is 1.8 mg/kg administered as an intravenous infusion every 21 days for 6 cycles. For previously untreated DLBCL: In combination with R-CHP. For relapsed/refractory DLBCL: In combination with bendamustine (90 mg/m²/day on Days 1 and 2) and rituximab (375 mg/m² on Day 1). Do not exceed 240 mg/cycle in limited experience with higher doses.
-
Administration: Administer as an intravenous infusion through a dedicated line with a 0.2- or 0.22-micron in-line filter. Initial dose: Over 90 minutes, monitor for infusion-related reactions during and for 90 minutes after. Subsequent doses: Over 30 minutes if prior infusion tolerated, monitor during and for 30 minutes after. Premedicate with antihistamine and antipyretic if not already done for rituximab. Reconstitute to 20 mg/mL with Sterile Water for Injection, then dilute to 0.72–2.7 mg/mL in compatible infusion bag (e.g., 0.9% NaCl, 0.45% NaCl, or 5% Dextrose). Do not mix with other drugs or administer as bolus/push.
-
Missed Dose: If a dose is missed, administer as soon as possible; adjust schedule to maintain 21-day intervals between doses.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Fatigue, diarrhea, pyrexia, nausea, decreased appetite
-
Peripheral neuropathy, anemia, thrombocytopenia, neutropenia
-
-
Serious Adverse Reactions:
-
Infusion-related reactions
-
Serious infections (e.g., sepsis, pneumonia)
-
Progressive multifocal leukoencephalopathy (PML)
-
Tumor lysis syndrome
-
-
-
Contraindications:
-
Hypersensitivity to Polatuzumab Vedotin or any component of the formulation.
-
Patients with active, severe infections.
-
-
Precautions:
-
Monitor blood counts regularly during treatment.
-
Avoid use during pregnancy; may cause fetal harm.
-
Discontinue treatment if grade ≥3 peripheral neuropathy develops.
-
Caution in patients with hepatic impairment.
-
Polatuzumab Vedotin Interactions
-
Concomitant use with strong CYP3A4 inhibitors or inducers may alter exposure to the cytotoxic component (monomethyl auristatin E).
-
Monitor for increased toxicity when used with other myelosuppressive agents.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.




Reviews
There are no reviews yet.