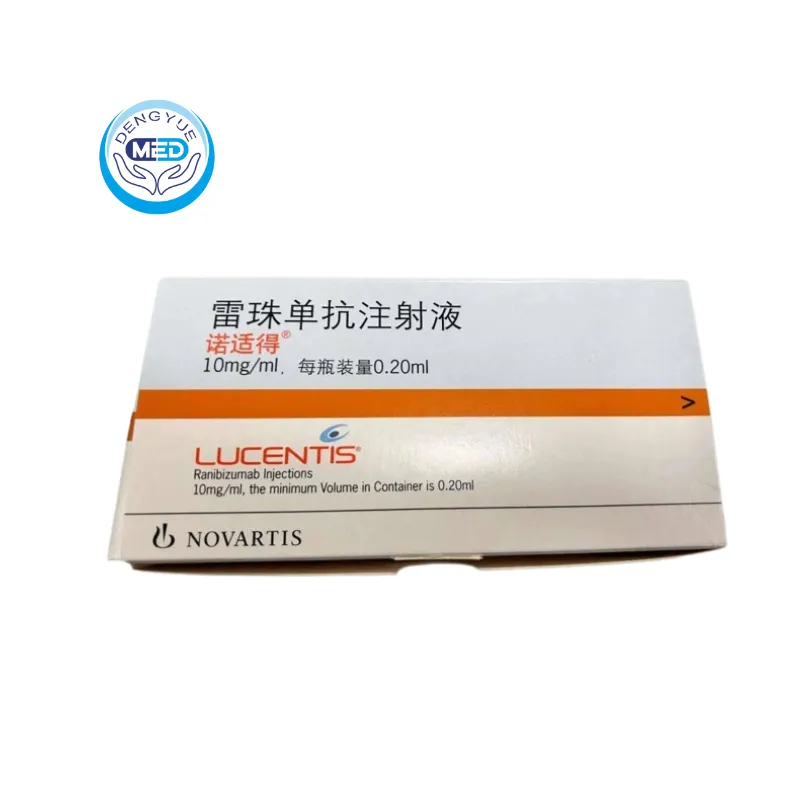
Lucentis (Ranibizumab) – AMD | HongKong DengYue Medicine
- Generic Name/Brand Name: Ranibizumab / Lucentis®
- Indications: AMD, DME, DR, RVO, mCNV
- Dosage Form: Injection
- Specification: 10mg × 1 syringe/box
Ranibizumab Application Scope
Ranibizumab is an anti-VEGF (vascular endothelial growth factor A) monoclonal antibody fragment used to treat ocular diseases caused by abnormal angiogenesis and vascular leakage.
It is mainly indicated for neovascular (wet) age-related macular degeneration (AMD), diabetic macular edema (DME), diabetic retinopathy (DR), macular edema following retinal vein occlusion (RVO), and myopic choroidal neovascularization (mCNV).

Ranibizumab Characteristics
-
Ingredients: Ranibizumab (Fab fragment of humanized monoclonal antibody against VEGF-A).
-
Properties: Sterile, clear, colorless to slightly yellow aqueous solution for intravitreal injection.
-
Packaging Specification: Prefilled syringes: 10mg × 1 syringe/box; single-use vials also available.
-
Storage: Store at 2–8 °C, protect from light, do not freeze.
-
Expiry Date: As indicated on the carton; shelf life typically 12–24 months.
-
Executive Standard: Ophthalmic biological product standard (refer to local NMPA / FDA / EMA).
-
Approval Number: As shown on local approval document.
-
Date of Revision: As printed on package insert.
-
Manufacturer: Novartis / Genentech (region dependent).
Guidelines for the Use of Lucentis
-
Dosage and Administration:
-
Administration: For intravitreal use only, under aseptic conditions by trained ophthalmologists.
-
Missed Dose: Administer as soon as possible, then continue regular schedule.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Conjunctival hemorrhage
-
Eye pain or irritation
-
Increased intraocular pressure
-
Vitreous floaters
-
Mild ocular inflammation
-
Transient blurred vision
-
-
Serious Adverse Reactions:
-
Endophthalmitis
-
Retinal detachment
-
Severe intraocular inflammation
-
Vitreous hemorrhage
-
Rare arterial thromboembolic events (stroke, myocardial infarction)
-
-
-
Contraindications:
-
Active ocular or periocular infection
-
Hypersensitivity to ranibizumab or its excipients
-
-
Precautions:
-
Maintain aseptic injection technique to minimize infection risk.
-
Monitor intraocular pressure immediately after injection.
-
Use caution in patients with recent arterial thromboembolic events.
-
Use during pregnancy or lactation only if potential benefit outweighs risk.
-
Do not mix with other drugs in the same syringe or vial.
-
Ranibizumab Interactions
-
No clinically significant systemic drug interactions reported.
-
Concurrent ocular procedures may increase inflammation or infection risk — manage with caution.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.
Related products
-

Bimzelx (Bimekizumab) – Psoriasis | HongKong DengYue Medicine
-

Cuprior (Trientrine Tetrahydrochloride) – Wilson’s Disease | HongKong DengYue Medicine
-

Mayzent (Siponimod) – Multiple Sclerosis | DengYueMed
-

UPLIZNA (Inebilizumab) – IgG4‑RD | HongKong DengYue Medicine
-

Dayvigo (Lemborexant Tablets) – NREM/REM | HongKong DengYue Medicine
-

OLUMIANT (Baricitinib) – Rheumatoid Arthritis | HongKong DengYue Medicine
-

NBP (Butylphthalide) – Stroke | HongKong DengYue Medicine
-

Jianmin (Atomoxetine) – ADHD | HongKong DengYue Medicine




Reviews
There are no reviews yet.