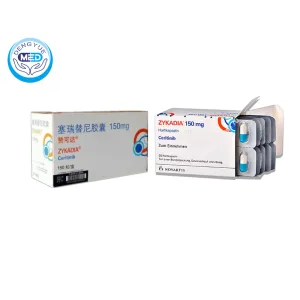
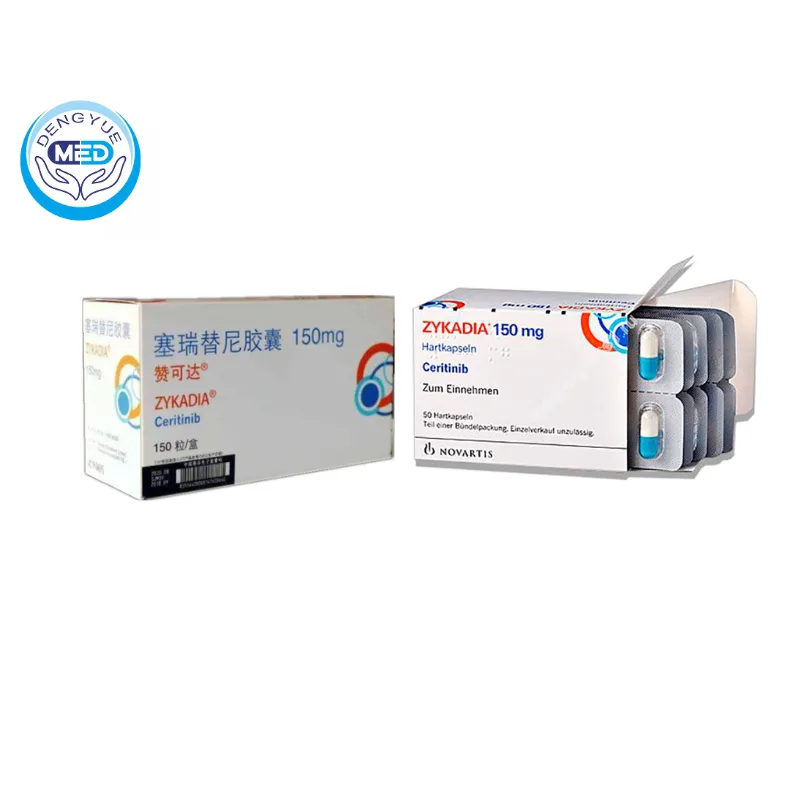
Zykadia (Ceritinib) – NSCLC | HongKong DengYue Medicine
- Generic Name/Brand Name: Ceritinib / Zykadia
- Indications: NSCLC
- Dosage Form: Capsule
- Specification: 150 mg x 150 capsules
Zykadia Application Scope
Zykadia is an oral ALK inhibitor used to treat ALK-positive metastatic non-small cell lung cancer (NSCLC).
Characteristics
-
Ingredients: Ceritinib
-
Properties: Tyrosine kinase inhibitor targeting ALK, ROS1, IGF-1R, and InsR; inhibits tumor cell proliferation and survival.
-
Packaging Specification: 150 mg film-coated tablets
-
Storage:
-
Store at 20°C to 25°C (68°F to 77°F)
-
Excursions permitted between 15°C and 30°C (59°F to 86°F)
-
-
Expiry Date: Refer to the packaging for the expiration date; use within the specified period.
-
Executive Standard: Refer to the prescribing information provided by the manufacturer.
-
Approval Number: FDA approval date: April 29, 2014
-
Date of Revision: Refer to the latest prescribing information for the most recent revision date
-
Manufacturer: Novartis Pharmaceuticals Corporation
Guidelines for the Use of Zykadia
-
Dosage and Administration:
-
Recommended Dose: 450 mg orally once daily with food until disease progression or unacceptable toxicity.
-
Administration: Swallow tablets whole; do not crush or chew.
-
-
Adverse Reactions:
-
Common (≥25%): Diarrhea, nausea, vomiting, abdominal pain, fatigue, decreased appetite.
-
Laboratory Abnormalities: Elevated liver enzymes (ALT, AST), hyperglycemia, increased lipase.
-
-
Contraindications: None listed.
-
Precautions:
-
Monitor liver function tests regularly.
-
Assess ECG for QT interval prolongation.
-
Monitor blood glucose levels in diabetic or hyperglycemic patients.
-
Zykadia Interactions
-
Drug Interactions:
-
Avoid concomitant use with strong CYP3A inhibitors or inducers.
-
Ceritinib is a CYP3A substrate and inhibitor; it may affect the metabolism of other drugs.
-
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.








Reviews
There are no reviews yet.