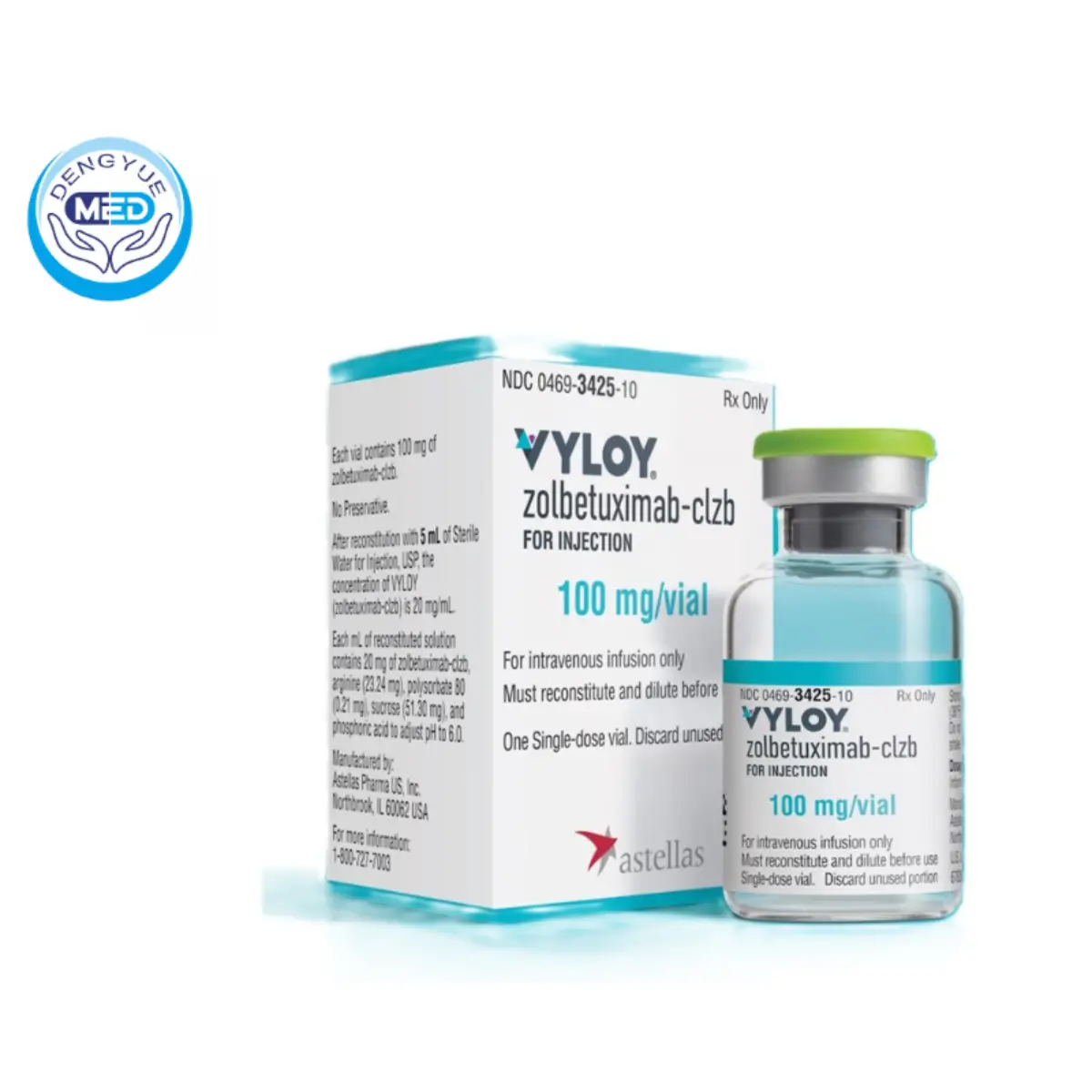
Zolbetuximab-Clzb For Injection|Stomach Cancer
- Generic Name/Brand Name: Zolbetuximab-Clzb For Injection/Willoway
- Indications: Stomach Cancer
- Dosage Form: Fluids
- Specification: 100 mg or 200 mg vials
Zolbetuximab-Clzb For Injection Application Scope
Zolbetuximab (Zolbetuximab-clzb) is a monoclonal antibody targeting CLDN18.2 (Claudin 18.2), a protein expressed in the cell membranes of certain tumor cells. It is primarily used in the treatment of cancers that express this protein, especially in gastrointestinal cancers.
- Gastric Cancer (GC):For 2-positive advanced or metastatic gastric cancer in patients who have not responded to conventional chemotherapy.
- Gastroesophageal Junction Cancer:Indicated for 2-positive tumors in the gastroesophageal junction.
- Pancreatic Cancer:Currently being investigated in clinical trials for 2-positive pancreatic cancer.
Zolbetuximab Package Insert
Ingredients:
- Active Ingredient:Zolbetuximab
- Inactive Ingredients:Sodium chloride, water for injection, histidine, and other stabilizers (may vary by manufacturer).
Properties:
- Mechanism of Action:Zolbetuximab-clzb binds specifically to the 2 protein expressed on tumor cells, initiating immune responses that lead to tumor cell destruction. This selective targeting makes it a valuable therapeutic option in CLDN18.2-positive cancers.
- Form:Monoclonal antibody solution for intravenous infusion.
- Physical Appearance:Clear, colorless to slightly yellow solution.
- Stability:Typically stable at refrigerated temperatures and should not be frozen.
Specification:
- Concentration:Commonly 25 mg/mL or 50 mg/mL solutions.
- Vial Size:100 mg or 200 mg vials, depending on manufacturer.
Packaging Specification:
- Packaging Type:Glass vials or pre-filled infusion bags.
- Vial Sizes:Commonly supplied in 100 mg or 200 mg vials.
- Secondary Packaging:Typically, one vial per carton along with instructions for use.
Storage:
- Storage Conditions:Store in a refrigerator at 2°C – 8°C (36°F – 46°F). Do not freeze.
- Shelf Life:Typically 24 months (2 years) from the manufacture date.
- Protect from Light:Keep in the original carton until ready for use.
Expiry Date:
- Four years
Executive Standard:
- Regulatory Compliance:Must comply with local and international regulatory standards such as the Chinese Pharmacopeia or FDA standards for biological products.
Approval Number:
- NMPA Approval Number:Specific approval number from the National Medical Products Administration (NMPA) or regional authority for marketing authorization.
Date of Revision:
- Revision Date:Date of the most recent product information revision or regulatory submission.
Manufacturer:
- Astellas
Guidelines For The Use Of Zolbetuximab-Clzb For Injection
Dosage and Administration:
- Dosage:Zolbetuximab-clzb is generally administered as an intravenous (IV) infusion.
- Initial Dose:Typically 800 mg/m² body surface area for the first dose.
- Subsequent Doses:After the initial dose, 600 mg/m² body surface area every two weeks.
- Infusion Duration:The infusion time for the first dose is typically 60 minutes, and can be reduced for subsequent doses if no significant infusion reactions are noted.
Adverse Reactions:
- Zolbetuximab Side Effects:
- Fatigue
- Nausea
- Diarrhea
- Fever
- Injection site reactions (e.g., redness, swelling)
- Serious Adverse Effects:
- Infusion-related reactions:Fever, chills, difficulty breathing, or hypotension.
- Severe allergic reactions (anaphylaxis):Monitor for signs during the first infusion.
- Gastrointestinal toxicity:Including gastrointestinal perforations in rare cases.
- Immune-related adverse effectssuch as hepatitis, pneumonitis, and colitis.
- Bone marrow suppression:Low white blood cell count, anemia, or thrombocytopenia.
Zolbetuximab-Clzb For Injection Medication Limitations
- Indication-specific:Only effective for 2-positive cancers. Diagnostic testing for CLDN18.2 expression is necessary prior to treatment.
- Combination Therapy:Often used in combination with chemotherapy agents like fluorouracil or oxaliplatin.
- Limited to advanced-stage cancers:Primarily for advanced or metastatic cancers that have progressed on other therapies.
Contraindications:
- Hypersensitivity:Contraindicated in patients who have known hypersensitivity to zolbetuximab or any of its components.
- Severe Infusion Reactions:Not recommended for patients who have had a severe allergic reaction to a previous dose.
Precautions:
- Infusion Monitoring:Patients should be monitored closely during the first infusion for potential infusion-related reactions. Premedication with antihistamines or corticosteroids may be required.
- Liver and Kidney Function:Monitor liver function tests regularly, as liver toxicity can occur in some patients.
- Pregnancy Category:Category C (potential risk to fetus) — should not be used during pregnancy unless the benefit outweighs the risk.
- Breastfeeding:It is recommended to avoid breastfeeding during treatment.
Zolbetuximab-Clzb For Injection Interactions
- Chemotherapy Interactions: Zolbetuximab-clzb may enhance the cytotoxicity of other chemotherapy agents, such as fluorouracil (5-FU) and cisplatin, making combination therapies more effective but also increasing the risk of side effects like myelosuppression.
- Immunosuppressive Agents:Co-administration with immunosuppressive drugs may exacerbate the risk of immune-related side effects.
- Radiation Therapy:When combined with radiation therapy, zolbetuximab can lead to an increased risk of gastrointestinal toxicity. Monitoring for side effects is critical.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine. It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.
Contact Us



Reviews
There are no reviews yet.