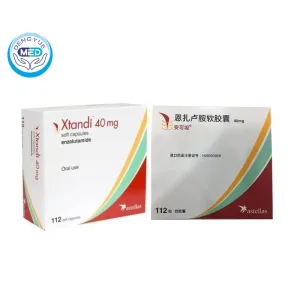
Xtandi (Enzalutamide Soft Capsules) – Prostate Cancer | HongKong DengYue Medicine
- Generic Name/Brand Name: Enzalutamide / XTANDI®
- Indications: Metastatic castration-resistant prostate cancer (mCRPC), nmCRPC, mCSPC and certain high-risk hormone-sensitive prostate cancers.
- Dosage Form: Soft gelatin capsule
- Specification: 40 mg x 112 soft capsules
Enzalutamide Soft Capsules Application Scope
Enzalutamide (XTANDI®) is indicated for adult men with prostate cancer in the following settings:
-
Metastatic castration-resistant prostate cancer (mCRPC)
-
Non-metastatic castration-resistant prostate cancer (nmCRPC)
-
Metastatic castration-sensitive prostate cancer (mCSPC)
-
High-risk non-metastatic hormone-sensitive prostate cancer (nmHSPC), as monotherapy or combined with androgen deprivation therapy
Enzalutamide Soft Capsules Characteristics
-
Ingredients: Enzalutamide – a nonsteroidal androgen receptor inhibitor.
-
Properties: Oral antiandrogen that blocks androgen receptor signaling to inhibit prostate cancer growth.
-
Packaging Specification:
White to off-white oblong soft gelatin capsules, imprinted with “ENZ.” Available in bottles of 120 capsules or blister packs (112 capsules; 28 per wallet). -
Storage: Store at 20–25 °C (68–77 °F), in a dry, well-ventilated place. Keep container tightly closed.
-
Expiry Date: As indicated on the packaging.
-
Executive Standard: Complies with FDA, EMA pharmacopeial and regulatory standards.
-
Approval Number: FDA-approved under NDA 203415 in August 2012 for mCRPC. Expanded approvals include nmCRPC (2018), mCSPC and nmHSPC (2019).
-
Date of Revision: Refer to latest label.
-
Manufacturer: Astellas Pharma (in partnership with Pfizer in certain regions).
Guidelines for the Use of Enzalutamide Soft Capsules
-
Dosage and Administration:
-
Recommended Dose: 160 mg orally once daily (e.g., four 40 mg soft capsules or two 80 mg tablets). Dose reduction (e.g., to 120/80 mg) may be considered for adverse effects.
-
Administration: Take orally, with or without food.
-
Missed Dose: If missed, take as soon as remembered unless the next scheduled dose is near. Do not double dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Fatigue, hot flushes, hypertension, falls, musculoskeletal pain.
-
Serious Adverse Reactions: Seizure (~0.4% incidence); neurological events (rare). If seizure occurs, discontinue enzalutamide.
-
-
Contraindications: During pregnancy – risk of fetal harm.
-
Precautions:
-
Monitor for seizures; avoid use in patients with predisposing factors.
-
Caution when prescribing with medications that lower seizure threshold.
-
Enzalutamide Soft Capsules Interactions
- Potent inducer of CYP3A4 and moderate inducer of CYP2C9 and CYP2C19. May reduce plasma levels of concomitant drugs metabolized by these enzymes (e.g. anticoagulants, some endocrine therapies).
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.




Reviews
There are no reviews yet.