Xanomeline And Trospium Chloride | Schizophrenia In Adults | HongKong DengYue Medicine
- Generic Name/Brand Name: Xanomeline and Trospium Chloride / Cobenfy
- Indications: Treatment Of Schizophrenia In Adults
- Dosage Form: Oral capsules
- Specification: 50 mg/20 mg, 100 mg/20 mg
Xanomeline And Trospium Chloride Application Scope
Cobenfy™ (xanomeline and trospium chloride), formerly KarXT, is an oral medication for the treatment of schizophrenia in adults.
Cobenfy combines xanomeline, a dual M1– and M4-preferring muscarinic receptor agonist, with trospium chloride, a muscarinic receptor antagonist that does not appreciably cross the blood-brain barrier, primarily confining its effects to peripheral tissues.
While the exact mechanism of action of Cobenfy is unknown, its efficacy is thought to be due to the agonist activity of xanomeline at M1 and M4 muscarinic acetylcholine receptors in the central nervous system.
Xanomeline And Trospium Chloride Characteristics
-
Ingredients:
-
Active Ingredients: Xanomeline (muscarinic M1/M4 receptor agonist), Trospium Chloride (peripherally acting muscarinic antagonist).
-
Inactive Ingredients: Specific excipients are not detailed in the available sources.
-
-
Properties: Oral capsules containing coated pellets.
-
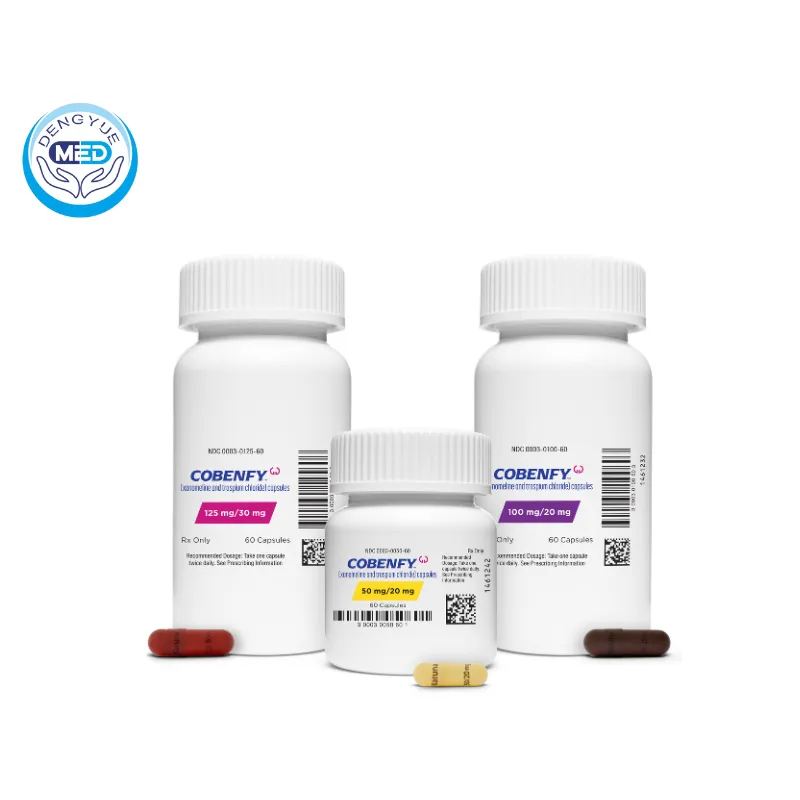
Packaging Specification: Available in capsule form with varying strengths:
-
50 mg/20 mg
-
100 mg/20 mg
-
125 mg/30 mg
-
-
Storage: Store at room temperature; specific storage conditions are not detailed in the available sources.
-
Expiry Date: Refer to the expiration date printed on the packaging.
-
Executive Standard: Refer to the prescribing information approved by regulatory authorities.
-
Approval Number: FDA Approval Number: NDA 216158.
-
Date of Revision: September 2024.
-
Manufacturer: Karuna Therapeutics, Inc.; marketed by Bristol Myers Squibb.
Guidelines for the Use of Xanomeline And Trospium Chloride
-
Dosage and Administration:
-
Initial Dose: 50 mg/20 mg capsule orally twice daily for at least two days.
-
Titration: Increase to 100 mg/20 mg capsule orally twice daily for at least five days.
-
Maximum Dose: 125 mg/30 mg capsule orally twice daily, based on patient tolerability and response.
-
Administration: Take orally at least one hour before or two hours after a meal.
-
-
Adverse Reactions:
-
Common (≥5%): Nausea, constipation, vomiting, hypertension, abdominal pain, diarrhea, tachycardia, dizziness, gastroesophageal reflux disease.
-
Serious: Urinary retention, increased heart rate, decreased gastric movement, angioedema.
-
-
Contraindications: Known hypersensitivity to xanomeline, trospium chloride, or any of the excipients.
-
Precautions:
-
Monitor for signs of urinary retention.
-
Assess heart rate and blood pressure periodically.
-
Use caution in patients with a history of gastrointestinal disorders.
-
Xanomeline And Trospium Chloride Interactions
-
Drug Interactions: No specific drug interaction studies have been conducted. Patients should inform their healthcare provider about all medications they are taking, including prescription and over-the-counter drugs, vitamins, and herbal supplements.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.









Reviews
There are no reviews yet.