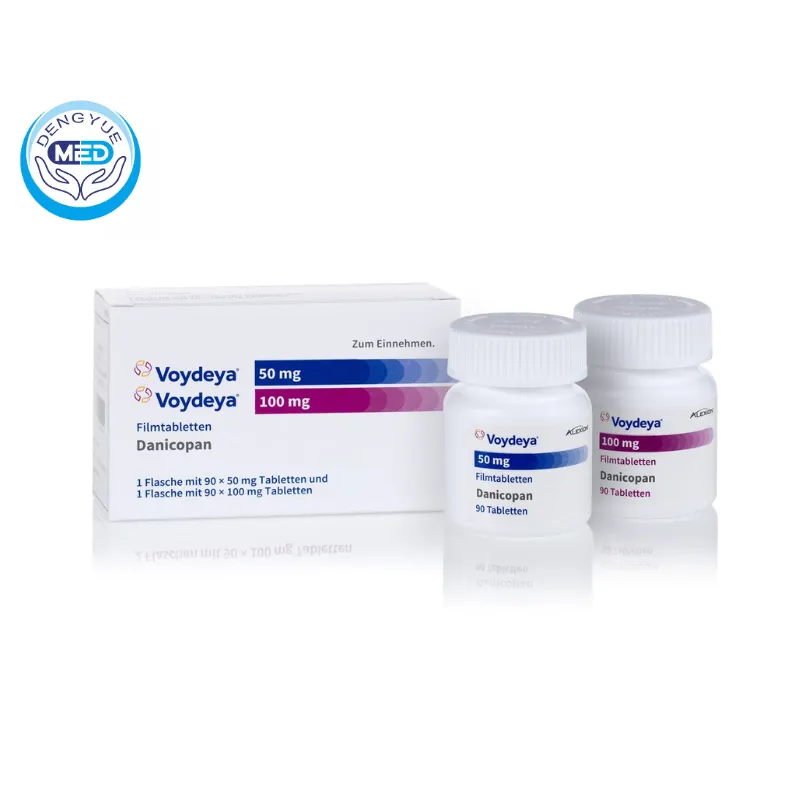
Voydeya (Danicopan) | Extravascular Hemolysis | HongKong DengYue Medicine
- Generic Name/Brand Name: Danicopan / Voydeya
- Indications: Extravascular hemolysis (EVH) in adults with paroxysmal nocturnal hemoglobinuria (PNH).
- Dosage Form: Oral film-coated tablets
- Specification: Available in 50 mg and 100 mg strengths.
Danicopan Application Scope

Voydeya is approved as an add-on therapy to ravulizumab or eculizumab for the treatment of extravascular hemolysis (EVH) in adults with paroxysmal nocturnal hemoglobinuria (PNH).
Danicopan Characteristics
-
Ingredients:
-
Active Ingredient: Danicopan
-
Excipients: Colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl methylcellulose acetate succinate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium lauryl sulfate.
-
Film Coating: Polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide.
-
-
Properties: White to off-white, round, film-coated tablets.
-
Packaging Specification: Available in bottles containing 90 tablets of either 50 mg or 100 mg strength.
-
Storage: Store at room temperature between 20°C and 25°C (68°F and 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F).
-
Expiry Date: Refer to the expiration date printed on the packaging.
-
Executive Standard: Approved by the U.S. Food and Drug Administration (FDA) on March 29, 2024.
-
Approval Number: NDA 218037.
-
Date of Revision: Refer to the latest prescribing information for the most recent revision date.
-
Manufacturer: Alexion Pharmaceuticals, Inc., a subsidiary of AstraZeneca.
Guidelines for the Use of Danicopan
-
Dosage and Administration:
-
Starting Dose: 150 mg taken orally three times daily (TID).
-
Dose Adjustment: If hemoglobin levels have not increased by more than 2 g/dL after 4 weeks, or if a transfusion was needed during the previous 4 weeks, the dose may be increased to 200 mg TID.
-
Administration: Can be taken with or without food. Swallow tablets whole; do not crush, chew, or split.
-
-
Adverse Reactions:
-
Common (≥10%): Headache.
-
Less Common (1% to 10%): Hypertension, vomiting.
-
Serious Adverse Reactions (reported in 5% of patients): Pancreatitis, cholecystitis, increased blood bilirubin.
-
-
Contraindications: Patients with unresolved serious infections caused by encapsulated bacteria, including Neisseria meningitidis, Streptococcus pneumoniae, or Haemophilus influenzae type B.
-
Precautions:
-
Voydeya increases susceptibility to serious, life-threatening, or fatal infections caused by encapsulated bacteria.
-
Ensure patients are vaccinated against Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae type B prior to initiating therapy.
-
Monitor liver function tests and lipid profiles during treatment.
-
Danicopan Interactions
- Drug Interactions:
-
Danicopan is an inhibitor of breast cancer resistance protein (BCRP) and P-glycoprotein (P-gp) transporters.
-
May increase plasma concentrations of BCRP and P-gp substrates, potentially requiring dose adjustments of concomitant medications such as rosuvastatin, fexofenadine, and tacrolimus.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.







Reviews
There are no reviews yet.