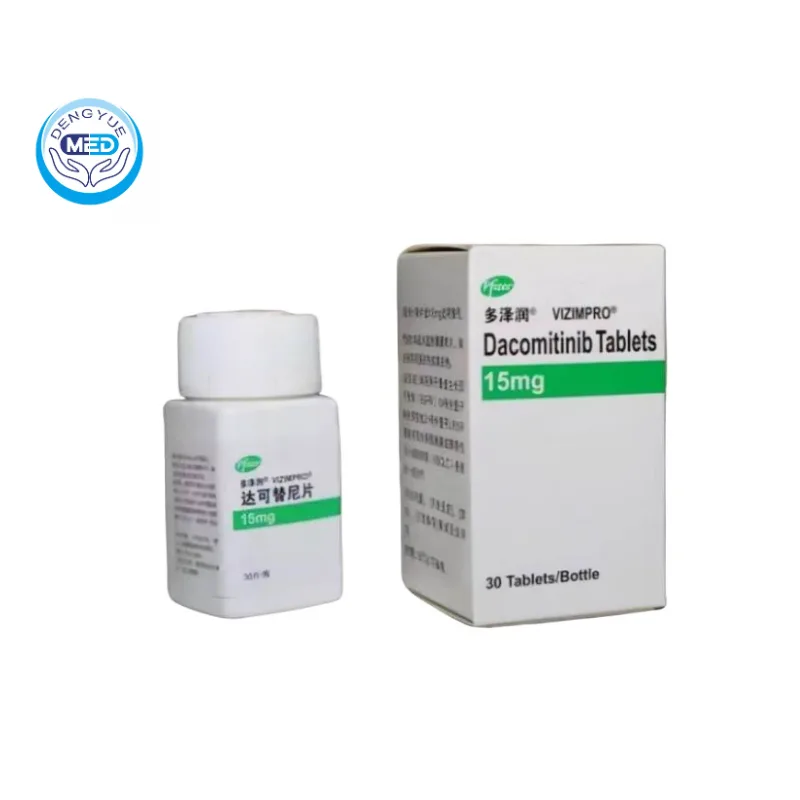
Vizimpro (Dacomitinib) – NSCLC | HongKong DengYue Medicine
- Generic Name/Brand Name: Dacomitinib/Vizimpro
- Indications: NSCLC (Lung Cancer)
- Dosage Form: Capsule
- Specification: 15 mg
Vizimpro Application Scope
Vizimpro is an oral, irreversible EGFR tyrosine kinase inhibitor used to treat certain types of metastatic non‑small cell lung cancer.

Characteristics
-
Ingredients: Dacomitinib
-
Properties:
-
Mechanism: selective, irreversible inhibitor of EGFR
-
Metabolism: CYP2D6 (major), CYP3A4 (minor), active metabolite O‑desmethyl‑dacomitinib
-
Elimination half‑life: ~70 hours; excreted mostly in feces (~79%)
-
-
Packaging Specification: Bottles of 30 tablets with child‑resistant caps (NDC-coded for each strength)
-
Storage: As per label: store at room temperature; specific details in full prescribing info
-
Expiry Date: Refer to the sticker on the bottle
-
Executive Standard: FDA prescription drug standards
-
Approval Number: Specific NDA number may vary; approved September 2018 in the US
-
Date of Revision: FDA label last revised December 2020; product approved September 2018
-
Manufacturer: Pfizer Inc.
Guidelines for the Use of Vizimpro
-
Dosage and Administration:
-
Starting dose: 45 mg orally once daily, with or without food, at the same daily time
-
Missed dose/vomiting: do not make up; continue next day
-
Dose reductions for toxicity: first reduction 30 mg, second 15 mg
-
Acid‑reducing medications:
-
Avoid proton pump inhibitors.
-
If using H₂‑blocker, take dacomitinib ≥ 6 hrs before or ≥ 10 hrs after
-
-
-
Adverse Reactions:
-
Common (>20%): diarrhea, rash, paronychia, stomatitis, dry skin, decreased appetite, weight loss, alopecia, cough, pruritus
-
Serious:
-
interstitial lung disease/pneumonitis (0.5%, fatal 0.3%)
-
grade 3–4 diarrhea (~11%, fatal 0.3%), rash (grade 3–4 in ~21%)
-
-
-
Contraindications: None explicitly listed
-
Precautions:
-
Monitor for ILD/pneumonitis; permanently stop if confirmed
-
Manage diarrhea and rash promptly; use anti‑diarrheals, topical/systemic skin treatments; adjust dose as needed
-
Can cause fetal harm: require pregnancy test and effective contraception during and ≥17 days after treatment
-
Avoid breastfeeding during treatment and for ≥17 days after the last dose
-
Vizimpro Interactions
-
Drug Interactions:
-
PPIs reduce absorption; avoid them
-
H₂‑blockers/antacids: separate dosing times
-
Inhibits CYP2D6: can increase plasma concentration of substrates significantly (e.g., dextromethorphan up 9‑fold)
-
Avoid concomitant use with drugs heavily metabolized by CYP2D6
-
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.








Reviews
There are no reviews yet.