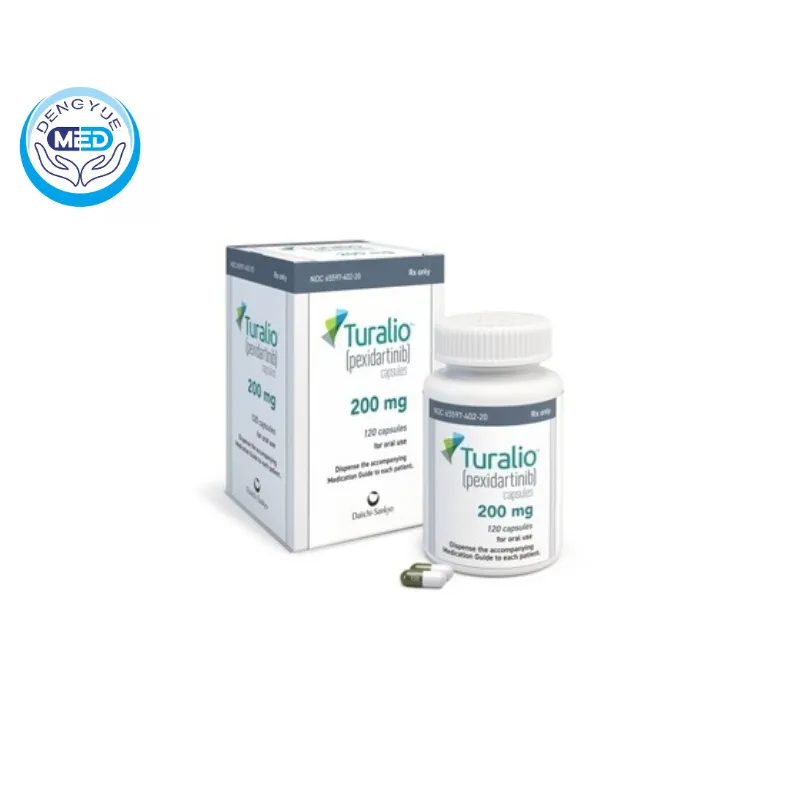
Turalio (Pexidartinib Capsules) – TGCT | HongKong DengYue Medicine
- Generic Name/Brand Name: Pexidartinib/Turalio
- Indications: TGCT
- Dosage Form: Oral capsules
- Specification: 200 mg × 120
Pexidartinib Capsules Application Scope
Pexidartinib is indicated for the treatment of symptomatic tenosynovial giant cell tumor (TGCT) associated with severe morbidity or functional limitations and not amenable to improvement with surgery.
Pexidartinib Capsules Characteristics
-
Ingredients: Pexidartinib hydrochloride (active ingredient)
-
Properties: Pexidartinib is a small-molecule tyrosine kinase inhibitor that selectively inhibits colony-stimulating factor-1 receptor (CSF1R), KIT, and FLT3-ITD. It reduces tumor growth by blocking macrophage proliferation and accumulation in TGCT.
-
Packaging Specification: Hard capsules, commonly available in 200 mg strength.
-
Storage: Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F). Protect from moisture and light.
-
Expiry Date: Refer to the product label; typically 24–36 months from the date of manufacture.
-
Executive Standard: In accordance with FDA/EMA approved drug standards or local pharmacopeia requirements.
-
Approval Number: U.S. FDA NDA 211810 (brand name: Turalio®). Local approval numbers vary by country.
-
Date of Revision: Latest prescribing information revised in 2025.
-
Manufacturer: Daiichi Sankyo, Inc.
Guidelines for the Use of Pexidartinib Capsules
-
Dosage and Administration:
-
Recommended Dose: 400 mg orally twice daily on an empty stomach (at least 1 hour before or 2 hours after a meal).
-
Administration: Swallow capsules whole with water. Do not open, crush, or chew.
-
Missed Dose: If a dose is missed, take the next scheduled dose at the regular time. Do not double dose to make up for the missed one.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Hair color changes (hypopigmentation)
-
Fatigue
-
Nausea, vomiting
-
Increased liver enzymes (ALT, AST)
-
Dysgeusia (taste changes)
-
Rash
-
Edema
-
-
Serious Adverse Reactions:
-
Hepatotoxicity (boxed warning: risk of serious and potentially fatal liver injury)
-
Anemia, neutropenia
-
QT prolongation
-
Severe hypersensitivity reactions
-
-
-
Contraindications:
-
Severe hepatic impairment
-
Known hypersensitivity to pexidartinib or any excipients
-
-
Precautions:
-
Monitor liver function tests (LFTs) prior to and during treatment
-
Avoid use with strong CYP3A inhibitors/inducers
-
Pregnancy: may cause fetal harm, effective contraception required
-
Breastfeeding: not recommended during treatment and for 1 week after last dose
-
Pexidartinib Capsules Interactions
-
CYP3A inhibitors (e.g., ketoconazole, clarithromycin): ↑ Pexidartinib exposure → ↑ toxicity risk
-
CYP3A inducers (e.g., rifampin, phenytoin): ↓ Pexidartinib exposure → reduced efficacy
-
Acid-reducing agents (e.g., PPIs, H2 blockers): may alter absorption
-
Avoid grapefruit and grapefruit juice (CYP3A interaction)
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.






Reviews
There are no reviews yet.