Truqap (Capivasertib) – Breast Cancer | HongKong DengYue Medicine
- Generic Name/Brand Name: Capivasertib / Truqap®
- Indications: Breast cancer
- Dosage Form: Oral tablets
- Specification: 160 mg, 200 mg × 64 tablets per box
Capivasertib Application Scope
Capivasertib, in combination with fulvestrant, is indicated for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced or metastatic breast cancer with one or more PIK3CA/AKT1/PTEN alterations, as detected by an FDA-approved test, following progression on at least one endocrine-based regimen in the metastatic setting or recurrence on or within 12 months of completing adjuvant therapy.
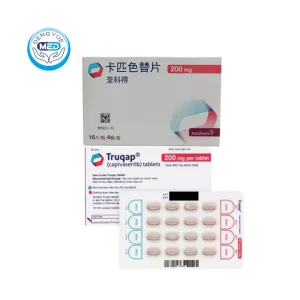
Capivasertib Characteristics
-
Ingredients: Capivasertib
-
Properties: Serine/threonine kinase inhibitor
-
Packaging Specification: 160 mg and 200 mg tablets
-
Storage: Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F)
-
Expiry Date: As per manufacturer labeling
-
Executive Standard: As per FDA and EMA guidelines
-
Approval Number: FDA: 218197; EMA: EMA/CHMP/1084/2024
-
Date of Revision: As per latest labeling updates
-
Manufacturer: AstraZeneca
Guidelines for the Use of Truqap
-
Dosage and Administration:
-
Recommended Dose: 400 mg orally twice daily (approximately 12 hours apart) with or without food, for 4 days followed by 3 days off. Continue until disease progression or unacceptable toxicity.
-
Administration: Oral tablets
-
Missed Dose: If a dose is missed, administer as soon as possible. Resume the dosing schedule from the most recently administered dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions (≥5% and >3% higher than placebo):
-
Injection site reactions (19%)
-
Decreased platelet count (12%)
-
Arthralgia (9%)
-
-
Serious Adverse Reactions:
-
Hypersensitivity reactions, including bronchospasm, diffuse erythema, facial swelling, urticaria, chills, and myalgias.
-
-
-
Contraindications: Patients with a history of serious hypersensitivity to it or any of the excipients in Truqap.
-
Precautions:
-
Hypersensitivity Reactions: Monitor patients for signs and symptoms. Discontinue use if reactions occur.
-
Platelet Count Monitoring: Monitor platelet counts during treatment.
-
Pregnancy and Lactation: Advise patients to inform their healthcare provider if they are pregnant, plan to become pregnant, are breastfeeding, or plan to breastfeed.
-
Capivasertib Interactions
-
Drug Interactions: It is metabolized by CYP3A4. Co-administration with strong CYP3A4 inhibitors or inducers may alter it plasma concentrations.
-
Food Interactions: It can be taken with or without food.
-
Laboratory Test Interactions: It may affect laboratory tests, including liver function tests.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.





Reviews
There are no reviews yet.