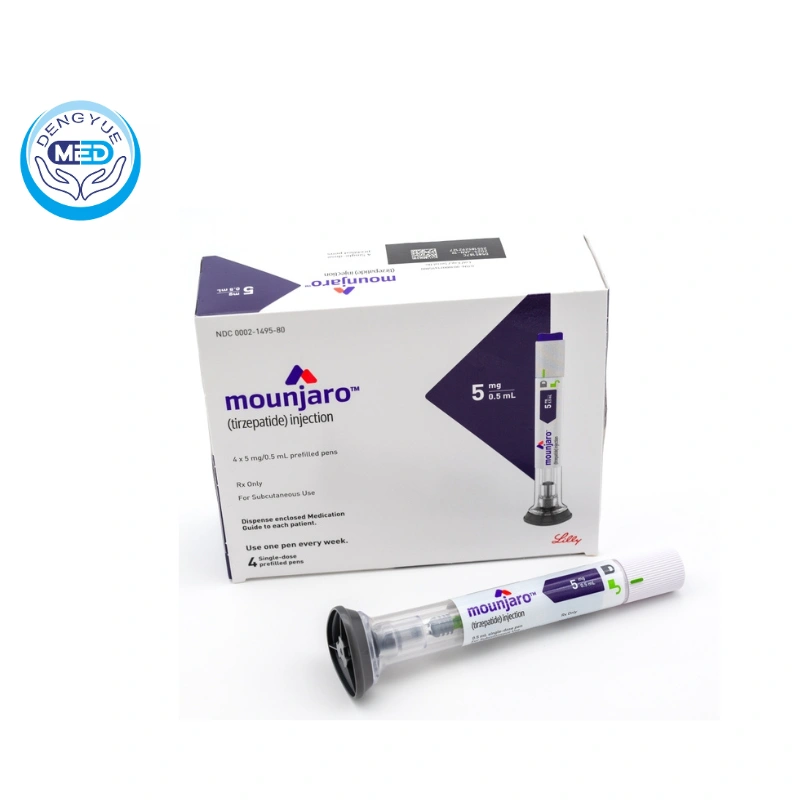
Tirzepatide|Type 2 Diabetes Mellitus|HongKong DengYue Medicine
- Generic Name/Brand Name: Tirzepatide/Mounjaro
- Indications: Type 2 Diabetes Mellitus
- Dosage Form: Subcutaneous injection (pre-filled pen)
- Specification: 2.5/5/7.5/10/12.5/15 mg
Tirzepatide Application Scope
- Primary Indications:
- Type 2 Diabetes Mellitus (T2DM): Approved for improving glycemic control in adults with T2DM, used alongside diet and exercise 15.
- Obesity/Overweight: In clinical trials, Tirzepatide demonstrated significant weight loss (up to 22.5% of body weight) in non-diabetic obese/overweight patients, though this indication is pending regulatory approval in some regions 111.
- Potential Future Uses: Under investigation for cardiovascular diseases, NASH (non-alcoholic steatohepatitis), and sleep apnea 15.
Tirzepatide Characteristics
- Ingredients: Synthetic dual agonist of GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic polypeptide) receptors.
- Properties: – Molecular Formula: C₂₂₅H₃₄₈N₄₈O₆₈- Molecular Weight: 4813.45 g/mol- Appearance: Not specified (typically a lyophilized powder for injection).
- Specification: Available in 5 mg, 10 mg, and 15 mg doses for weekly subcutaneous injection.
- Packaging Specification: Pre-filled pens or vials (exact packaging details not specified in sources).
- Storage: Store refrigerated (2–8°C) before use; may be kept at room temperature for a limited time after first use.
- Expiry Date: Typically 24 months from manufacturing (check product labeling for exact dates).
- Executive Standard: Manufactured under FDA (U.S.), EMA (EU), and NMPA (China) regulations.
- Approval Number: – U.S. FDA Approval: May 2022 (Mounjaro®) .- China NMPA Submission: September 2022 (under review) .
- Date of Revision: Latest clinical trial updates as of 2024 (SURPASS-CVOT expected in 2024).
- Manufacturer: Eli Lilly and Company
Guidelines For The Use Of Tirzepatide
- Dosage and Administration: – Starting Dose: 2.5 mg once weekly, titrated every 4 weeks to 5 mg, 10 mg, or 15 mg based on glycemic response and tolerability.- Administration: Subcutaneous injection in the abdomen, thigh, or upper arm.
- Adverse Reactions: Most common: Nausea, vomiting, diarrhea, constipation, decreased appetite, dyspepsia, abdominal pain (usually mild to moderate and transient).Less common: Hypoglycemia (when combined with insulin/SGLT-2 inhibitors), pancreatitis risk (rare) .
- Contraindications: – Personal/family history of medullary thyroid carcinoma (MTC)- Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)- Severe gastrointestinal disease (e.g., gastroparesis) .
- Precautions: – Monitor for pancreatitis, gallbladder disease, renal impairment, and diabetic retinopathy complications– Not recommended in pregnancy or breastfeeding (insufficient safety data).
Tirzepatide Interactions
Drug Interactions: – Insulin/Sulfonylureas: Increased risk of hypoglycemia (dose adjustment may be needed)
Oral Medications: Delayed absorption possible (take oral drugs 1 hour before Tirzepatide)
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.
Contact Us


Reviews
There are no reviews yet.