Teysuno (Tegafur Gimeracil and Oteracil Potassium) – Gastric Cancer | HongKong DengYue Medicine
- Generic/Brand Name: Tegafur Gimeracil and Oteracil Potassium Capsules / Teysuno®
- Indications: Metastatic Gastric Cancer, Stomach Cancer
- Dosage Form: Capsules
- Specification: 20mg × 42 Capsules/box
Tegafur Capsules Application Scope
Tegafur Gimeracil and Oteracil Potassium Capsules are used for the treatment of unresectable locally advanced or metastatic gastric cancer.
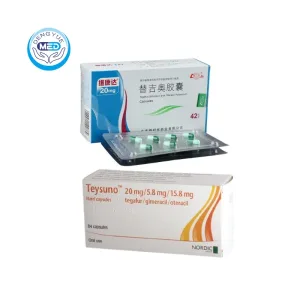
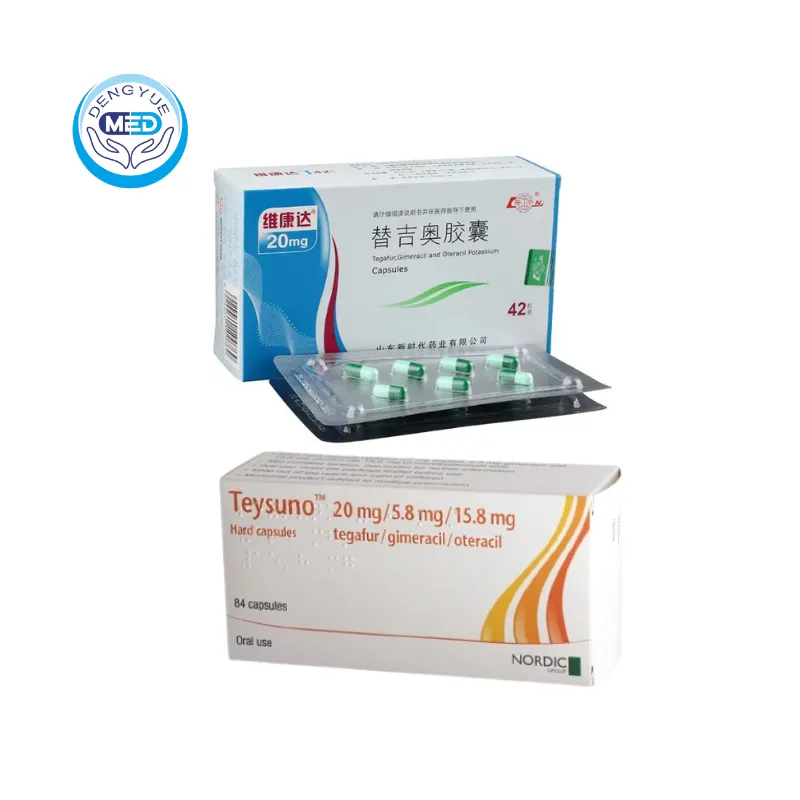
Features of Tegafur Gimeracil and Oteracil Potassium Capsules
Ingredients: Compound preparation, each capsule contains: Tegafur 20mg, Gemcitrimidine 5.8mg, Potassium otilazepate 19.6mg.
Characteristics: Treatment of gastric cancer
Specification: 20mg
Packing specification: Aluminum plastic packing, 42 capsules/box.
Storage: airtight, room temperature (10-30 ℃) storage.
Effective period: 24 months
Approval number: State Drug License H20080802
Revision date According to the latest drug instruction.
Manufacturer: Shandong New Era Pharmaceutical
Guidelines for the use of Tegafur Capsules
Dosage and administration: Take orally twice daily, after breakfast and dinner, for 28 consecutive days, with 14 days of rest, as a treatment cycle. Administer the drug until the patient’s condition deteriorates or is intolerable.
Adverse reactions: Gastrointestinal symptoms such as loss of appetite, nausea, vomiting, and diarrhea.
Restrictions on the use of Tegafur Gimeracil and Oteracil Potassium Capsules
Contraindications: contraindicated in patients with a history of severe allergy to the constituents of Tegafur capsules, contraindicated in patients with severe myelosuppression, severe renal function abnormalities
Interactions with Tegafur Gimeracil and Oteracil Potassium Capsules
Gimepiridine in the drug can inhibit the catabolism of 5-FU in the combined drug, causing a significant increase in the concentration of 5-FU in the blood.
Pay attention to the observation of the patient’s state, and take appropriate measures such as reducing the dosage or stopping the drug if abnormalities are found.
Side effects are mutually reinforcing. It may potentiate the action of bicoumarin and lead to abnormalities in coagulation. Before starting treatment, inform your doctor of all medications you are using.
Note: If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine. It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.
Related products
-

Alglucosidase Alfa – Pompe Disease
-

Proglycem Diazoxide | Hyperinsulinemic Hypoglycemia | DengYueMedicine
-

Enspryng Satralizumab | Optic Nerve Myelitis Spectrum Disorders
-

Spesolimab Injection | Psoriatic Arthritis Treatment
-

Thiamazole Cream | Anti Hyperthyroidism
-

ENHERTU (Trastuzumab Deruxtecan) – HER2-driven cancers | HongKong DengYue Medicine
-

Ozamode/Ozanimod Hydrochloride Capsules
-

Aidixi Disitamab Vedotin Of Injection | HER2-Overexpressing | DengYueMedicine




Reviews
There are no reviews yet.