TECVAYLI (Teclistamab) – RRMM | HongKong DengYue Medicine
- Generic Name/Brand Name: Teclistamab/TECVAYLI
- Indications: Relapsed or refractory multiple myeloma
- Dosage Form: Sterile solution for subcutaneous injection
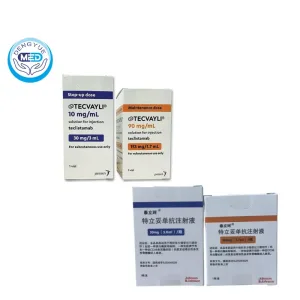
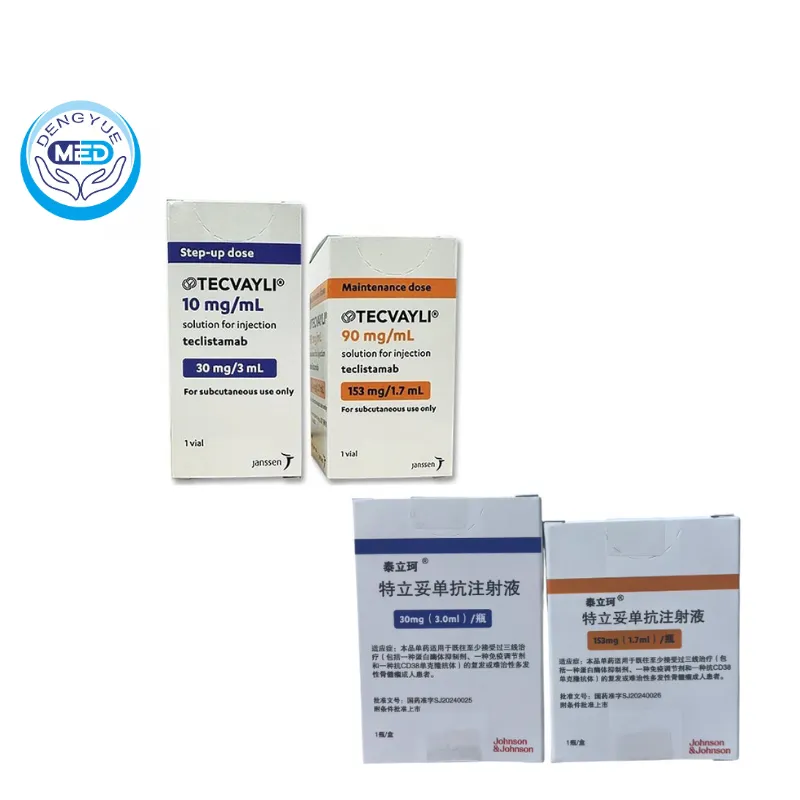
- Specification: 30 mg/3.0 mL × 1 vial (10 mg/mL); 153 mg/1.7 mL × 1 vial (90 mg/mL)
TECVAYLI Teclistamab Application Scope
TECVAYLI (teclistamab) is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least three prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy.
TECVAYLI Teclistamab Characteristics
-
Ingredients: Teclistamab
-
Properties: The solution is sterile, preservative-free, clear to slightly opalescent, colorless to light yellow
-
Packaging Specification: Two vial strengths: 30 mg / 3.0 mL vial (10 mg/mL); 153 mg / 1.7 mL vial (90 mg/mL)
-
Storage: Refrigerate at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light
-
Expiry Date: The expiry date is specified on the packaging
-
Executive Standard: Manufactured under compliance with applicable Good Manufacturing Practice (GMP) standards and approved by regulatory authorities (e.g., FDA, EMA)
-
Approval Number: Varies by region. Please refer to the local product packaging and labeling
-
Date of Revision: Please refer to the latest official prescribing information for the most recent revision date
-
Manufacturer: Janssen Inc.
Guidelines for the Use of TECVAYLI Teclistamab
-
Dosage and Administration:
-
Recommended Dose:
-
Begin with a step-up dose of 0.06 mg/kg given subcutaneously on Day 1.
-
Give a second step-up dose of 0.3 mg/kg subcutaneously on approximately Day 3 (about 2 to 4 days after the first step-up dose).
-
Administer the first full treatment dose of 1.5 mg/kg subcutaneously on approximately Day 5 (about 2 to 4 days after the second step-up dose).
-
After the first full treatment dose, continue with 1.5 mg/kg once weekly as maintenance therapy.
-
For patients who achieve a complete response or better and maintain that response for at least six months, the dosing frequency may be reduced to 1.5 mg/kg every two weeks if clinically appropriate.
-
-
Administration: For subcutaneous injection only. Administer in the abdomen (except for the 2-inch area around the navel) or thigh. Rotate injection sites.
-
Missed Dose: If a dose is missed, administer the missed dose as soon as possible. Thereafter, resume dosing at the regular scheduled time. Consult the prescribing physician for guidance.
-
-
Adverse Reactions:
-
Common Adverse Reactions: From MajesTEC-1 data: neutropenia, anemia, thrombocytopenia, lymphopenia, leukopenia; hypogammaglobulinemia; cytokine release syndrome (CRS); fatigue; infections; musculoskeletal pain, etc.
-
Serious Adverse Reactions:
-
Cytokine release syndrome (CRS) can be life-threatening.
-
Neurologic toxicities, including immune effector cell-associated neurotoxicity syndrome (ICANS).
-
Severe infections (including opportunistic) due to immunosuppression.
-
Significant cytopenias (e.g., neutropenia grade 3/4).
-
-
-
Contraindications: Hypersensitivity to teclistamab or any of the excipients.
-
Precautions:
-
Monitor for CRS; use step-up dosing to mitigate risk.
-
Monitor neurologic symptoms/ICANS. Active infection must be treated or controlled before administration.
-
Vaccination considerations: avoid live vaccines during treatment; prophylaxis for certain viruses (e.g., herpes zoster) may be recommended per institutional guidelines.
-
Special care in elderly patients; renal/hepatic impairment if data available; local morbidity of immune suppression.
-
TECVAYLI Interactions
-
No formal drug-drug interaction studies have shown clinically relevant interactions due to metabolic pathways (as a monoclonal antibody, it will not be metabolized by CYP enzymes) in published data. (Antibodies generally less prone to CYP interactions.)
-
Concomitant immunosuppressive drugs may increase the risk of infection.
-
Vaccines: live vaccines should not be used. Other vaccines may have reduced efficacy.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.








Reviews
There are no reviews yet.