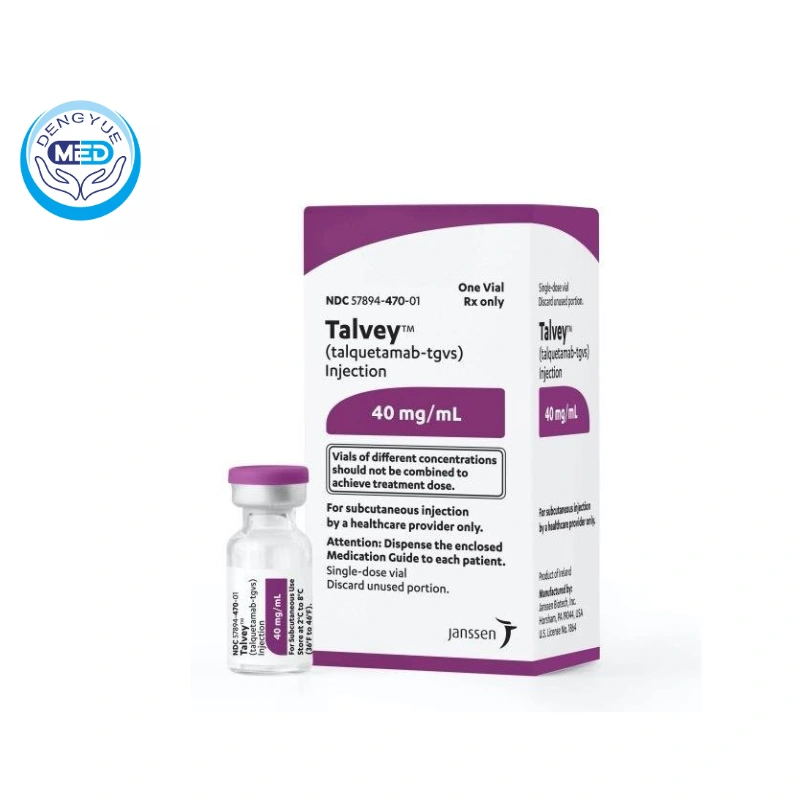
Talquetamab-tgvs|Multiple Myeloma|HongKong DengYue Medicine
- Generic Name/Brand Name: Talquetamab-tgvs/Talvey
- Indications: Multiple Myeloma
- Dosage Form: light yellow solution
- Specification: 3 mg/1.5 mL (2 mg/mL) or 40 mg/1 mL (40 mg/mL)
Talquetamab-tgvs Application Scope
Talquetamab-tgvs, marketed under the brand name TALVEY™, is a bispecific GPRC5D-directed CD3 T-cell engager indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
Characteristics of Talquetamab-tgvs
-
Ingredients: Talquetamab is a humanized immunoglobulin G4-proline, alanine, alanine (IgG4-PAA) bispecific antibody directed against G protein-coupled receptor family C group 5 member D (GPRC5D) and the cluster of differentiation 3 (CD3) receptors.
-
Properties: Talquetamab-tgvs binds to GPRC5D on multiple myeloma cells and CD3 on T cells, activating T cells to release cytokines and lyse multiple myeloma cells.
-
Specification: Available in single-dose vials containing either 3 mg/1.5 mL (2 mg/mL) or 40 mg/1 mL (40 mg/mL) of talquetamab-tgvs.
-
Packaging Specification: Supplied as a sterile, preservative-free, colorless to light yellow solution in single-dose vials.
-
Storage: Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze or shake.
-
Expiry Date: Refer to the expiration date printed on the packaging.
-
Executive Standard: Approved under accelerated approval based on response rate and durability of response. Continued approval may be contingent upon verification and description of clinical benefit in confirmatory trials.
-
Approval Number: FDA approval granted on August 9, 2023.
-
Date of Revision: Refer to the latest prescribing information for the most current revision date.
-
Manufacturer: Janssen Biotech, Inc.
Guidelines for the Use of Talquetamab-tgvs:
-
Dosage and Administration:
-
Step-Up Dosing Schedule:
-
Step-Up Dose 1: Administer 0.01 mg/kg subcutaneously on Day 1.
-
Step-Up Dose 2: Administer 0.06 mg/kg subcutaneously on Day 4.
-
First Treatment Dose: Administer 0.4 mg/kg subcutaneously on Day 7, followed by 0.4 mg/kg weekly thereafter.
-
-
Alternative Biweekly Dosing Schedule:
-
Step-Up Dose 1: Administer 0.01 mg/kg subcutaneously on Day 1.
-
Step-Up Dose 2: Administer 0.06 mg/kg subcutaneously on Day 4.
-
Step-Up Dose 3: Administer 0.3 mg/kg subcutaneously on Day 7.
-
First Treatment Dose: Administer 0.8 mg/kg subcutaneously on Day 10, followed by 0.8 mg/kg every two weeks thereafter.
-
-
Administer pre-treatment medications to reduce the risk of cytokine release syndrome (CRS).
-
-
Adverse Reactions:
-
Common adverse reactions include CRS, skin-related toxicities, dysgeusia, nail disorders, musculoskeletal pain, fatigue, and weight loss.
-
Serious adverse reactions may include infections, hepatotoxicity, and neurologic toxicity, including immune effector cell-associated neurotoxicity syndrome (ICANS).
-
-
Contraindications:
-
No contraindications have been identified based on the current prescribing information.
-
-
Precautions:
-
Monitor patients for signs and symptoms of CRS and administer supportive care as needed.
-
Monitor for signs of neurologic toxicity, including ICANS.
-
Advise females of reproductive potential to use effective contraception during treatment and for 3 months after the last dose.
-
Talquetamab-tgvs Interactions
-
Drug Interactions:
-
Talquetamab-tgvs may interact with CYP450 substrates; monitor patients for potential interactions.
-
Consult a healthcare provider for a comprehensive list of potential drug interactions.
-
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.





Reviews
There are no reviews yet.