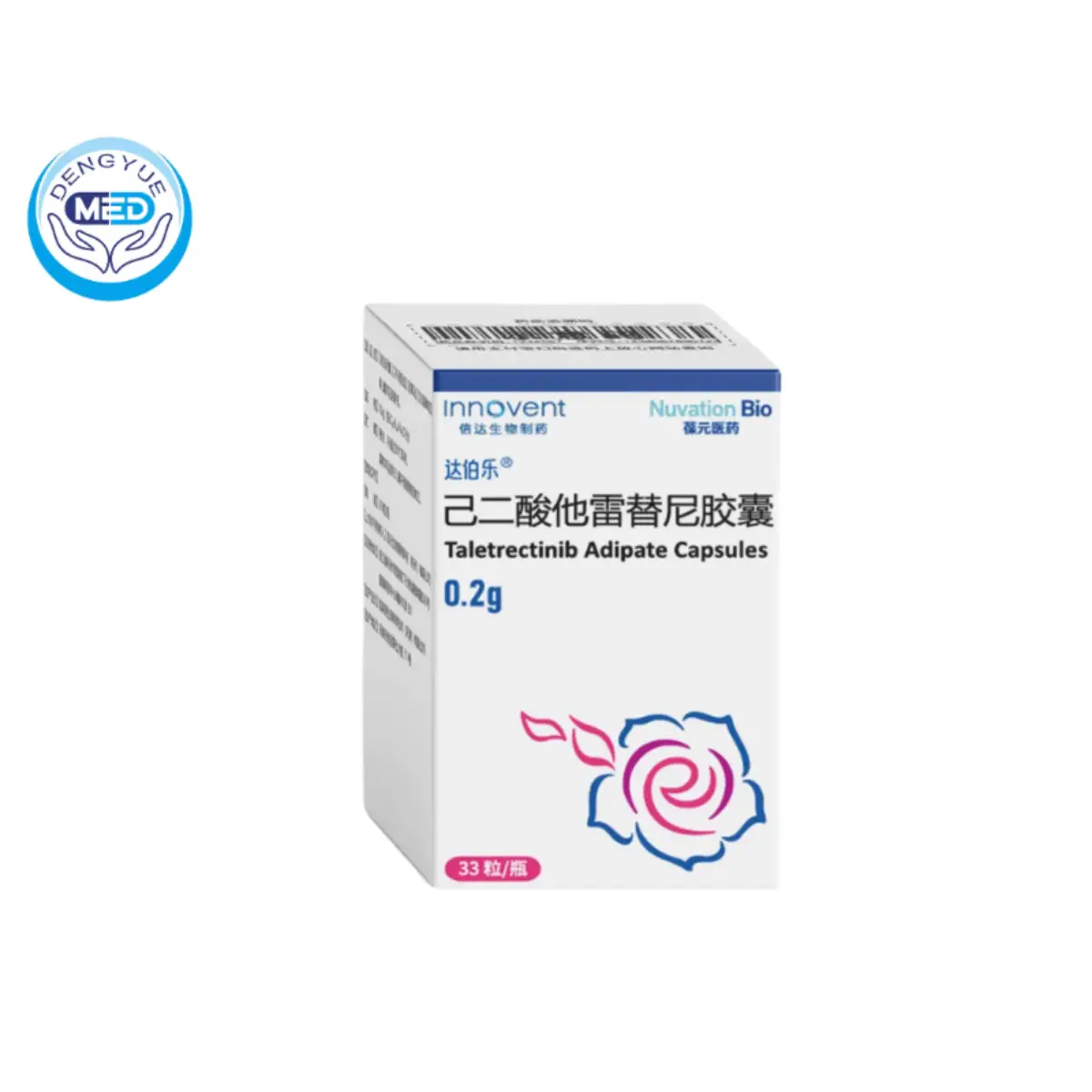
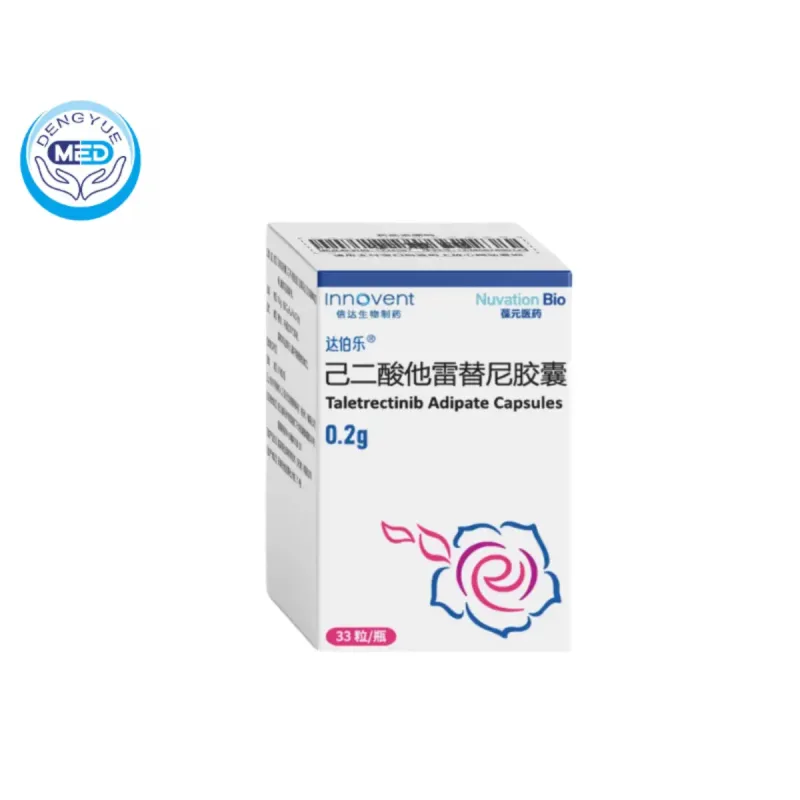
Taletrectinib Adipate Capsules|Non–small cell lung cancer
- Generic Name/Brand Name: Taletrectinib Adipate Capsules/Innovent
- Indications: Non–small cell lung cancer
- Dosage Form: Capsules
- Specification: 200 mg
Taletrectinib Adipate Capsules Application Scope
Taletrectinib adipate capsules, marketed as DOVBLERON®, are an oral tyrosine kinase inhibitor (TKI) targeting ROS1 and neurotrophic tyrosine receptor kinases (NTRK) 1, 2, and 3.
They are primarily indicated for the treatment of adult patients with locally advanced or metastatic ROS1-positive non–small cell lung cancer (NSCLC).
In December 2024, China’s National Medical Products Administration (NMPA) approved taletrectinib for patients who have progressed after prior ROS1 TKI therapy.
Characteristics of Taletrectinib Adipate Capsules
- Ingredients: The active ingredient is taletrectinib adipate.
- Properties: Taletrectinib is a selective inhibitor of ROS1 and NTRK tyrosine kinases, designed to impede tumor growth driven by these targets.
- Specification: Each capsule contains 200 mg of taletrectinib (calculated as C₂₃H₂₄FN₅O).
- Packaging Specification: Specific packaging details are not provided in the available sources.
- Storage: Store in a cool, dry place, away from direct sunlight.
- Expiry Date: Refer to the packaging for the expiration date.
- Executive Standard: Complies with the standards set by the NMPA.
- Approval Number: Specific approval numbers can be found on the official NMPA website or the product packaging.
- Date of Revision: Refer to the packaging or accompanying documentation for the latest revision date.
- Manufacturer: Developed collaboratively by AnHeart Therapeutics and Innovent Biologics.
Guidelines for the Use of Taletrectinib Adipate Capsules
- Dosage and Administration: The recommended dosage and administration schedule should be determined by a healthcare professional experienced in cancer treatment.
- Adverse Reactions: Common adverse reactions may include gastrointestinal disturbances, fatigue, and elevated liver enzymes. Patients should be monitored regularly for these effects.
- Contraindications: Contraindications include known hypersensitivity to taletrectinib or any component of the formulation.
- Precautions: Caution is advised in patients with hepatic impairment, and regular liver function tests are recommended during treatment.
Taletrectinib Adipate Capsules Drug Interactions
Taletrectinib may interact with other medications metabolized by the liver.
Patients should inform their healthcare provider of all medications they are taking, including over-the-counter drugs and supplements, to assess potential interactions.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.






Reviews
There are no reviews yet.