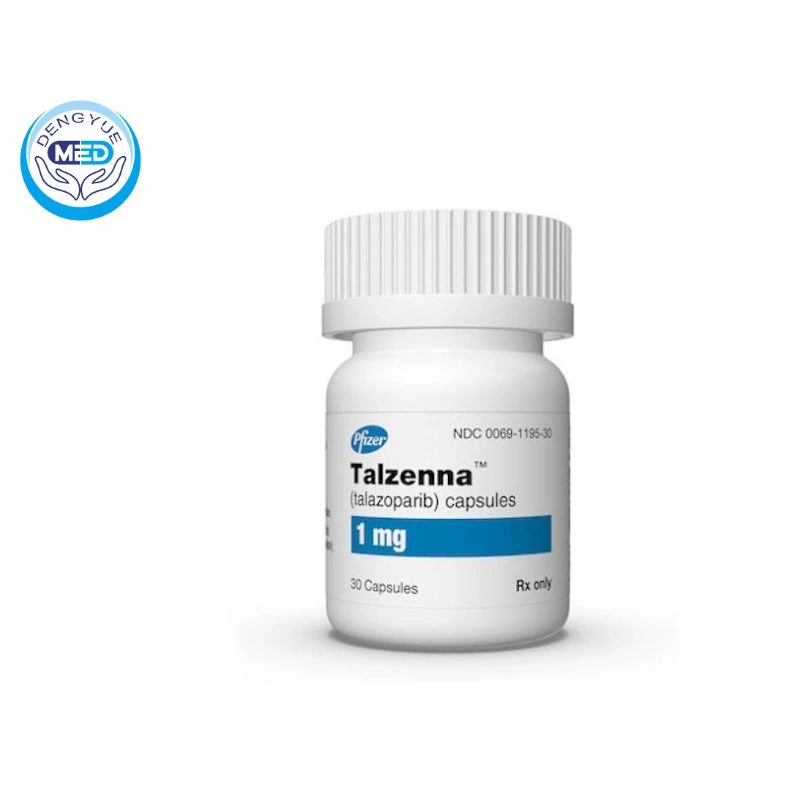
Talzenna (Talazoparib)|Breast Cancer|HongKong DengYue Medicine
- Generic Name/Brand Name: Talazoparib
- Indications: Breast Cancer
- Dosage Form: Capsules
- Specification: 30 capsules/1mg
Talazoparib Application Scope
Talazoparib, marketed under the brand name Talzenna, is an orally administered poly ADP ribose polymerase (PARP) inhibitor used in the treatment of certain cancers.
-
Breast Cancer: Talzenna is indicated for the treatment of adults with deleterious or suspected deleterious germline BRCA-mutated (gBRCAm), HER2-negative locally advanced or metastatic breast cancer.
-
Prostate Cancer: In combination with enzalutamide, talazoparib is approved for the treatment of homologous recombination repair (HRR) gene-mutated metastatic castration-resistant prostate cancer (mCRPC) in adults.
Talazoparib Characteristics
-
Ingredients: Each capsule contains talazoparib tosylate as the active ingredient.
-
Properties: Talzenna inhibits PARP enzymes, leading to DNA damage accumulation and cell death, particularly in cancer cells with BRCA mutations.
-
Specification: Available in capsule form for oral administration.
-
Packaging Specification: Supplied in bottles containing 30 capsules.
-
Storage: Store at room temperature, 20°C to 25°C (68°F to 77°F).
-
Expiry Date: Refer to the packaging for the expiration date.
-
Executive Standard: Approved by regulatory authorities such as the U.S. FDA and the European Medicines Agency (EMA).
-
Approval Number: Varies by country; consult local regulatory information.
-
Date of Revision: Refer to the latest prescribing information for the most recent revision date.
-
Manufacturer: Pfizer Inc.
Guidelines for the Use of Talazoparib
-
Dosage and Administration: The recommended dose is 1 mg taken orally once daily, with or without food. Dose adjustments may be necessary for patients with renal impairment.
-
Adverse Reactions: Common adverse reactions include fatigue, anemia, nausea, neutropenia, headache, thrombocytopenia, vomiting, alopecia, diarrhea, and decreased appetite.
-
Contraindications: None specified.
-
Precautions: Monitor blood counts regularly due to the risk of myelosuppression. Advise patients of the potential risk to a fetus and to use effective contraception during treatment.
Talazoparib Interactions
- Drug Interactions: Co-administration with P-glycoprotein (P-gp) or breast cancer resistance protein (BCRP) inhibitors may increase Talzenna concentrations.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.





Reviews
There are no reviews yet.