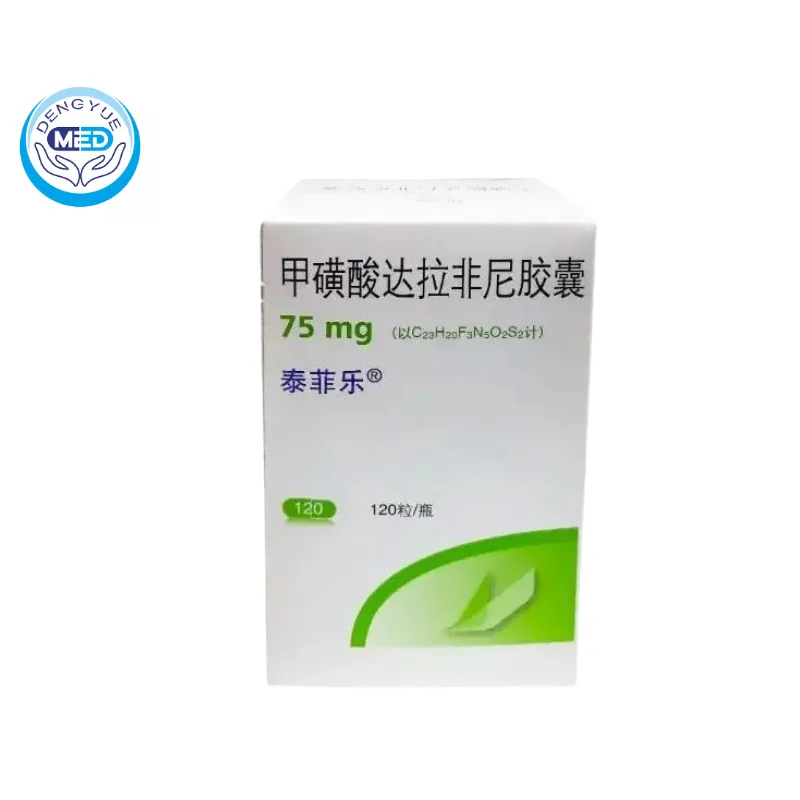
Tafinlar (Dabrafenib Mesylate) – Melanoma & Lung Cancer | DengYue Medicine
- Generic Name/Brand Name: Dabrafenib Mesylate/Tafinlar
- Indications: NSCLC, Metastatic Melanoma, Solid Tumors, Alone or with Trametinib.
- Dosage Form: Oral Capsules
- Specification: 75 mg x 120 capsules
Dabrafenib Mesylate Application Scope
Dabrafenib Mesylate is indicated for the treatment of patients with BRAF V600 mutation-positive unresectable or metastatic melanoma.
It is commonly administered in combination with Trametinib (a MEK inhibitor) to improve therapeutic efficacy and delay resistance.

Dabrafenib Mesylate Characteristics
-
Ingredients:
Dabrafenib MesylateProperties:
Oral kinase inhibitor targeting mutated BRAF proteins, interfering with MAPK pathway signaling to inhibit tumor growth.Packaging Specification:
Capsules of 50 mg and 75 mgStorage:
Store in a cool, dry place at 20°C to 25°C, protected from moisture and light.Expiry Date:
Refer to packaging labelExecutive Standard:
Over-the-counter OTCApproval Number:
H20190067Date of Revision:
2019Manufacturer:
Novartis Pharma Stein AG (Novartis Pharmaceuticals, Switzerland)Packaging Enterprise:
Novartis Pharmaceuticals (Beijing) Co., Ltd.
Guidelines for the Use of Dabrafenib Mesylate
-
Dosage and Administration:
-
Recommended Dose: 150 mg orally twice daily, approximately 12 hours apart, taken on an empty stomach (at least 1 hour before or 2 hours after a meal). Capsules should be swallowed whole without chewing, opening, or crushing.
In combination therapy with Trametinib, Dabrafenib dosing remains the same. Trametinib is administered once daily at 2 mg orally, also on an empty stomach.
-
-
Adverse Reactions:
-
Common Adverse Reactions (≥5% and >3% higher than placebo):
-
Skin and subcutaneous tissue disorders: alopecia, rash (erythema, papules, commonly on head, neck, trunk), palmar-plantar erythrodysesthesia syndrome (redness, swelling, pain in palms and soles)
-
Metabolic abnormalities: hypophosphatemia, hyperglycemia
-
General symptoms: fever, chills, fatigue, headache
-
Gastrointestinal: nausea, vomiting, diarrhea, abdominal pain
-
-
Serious Adverse Reactions:
-
Cutaneous squamous cell carcinoma and keratoacanthoma (approx. 25% incidence, mainly within first 6 months)
-
Hemorrhage (e.g., epistaxis, hemoptysis, gastrointestinal bleeding)
-
Venous thromboembolism (pulmonary embolism, deep vein thrombosis)
-
Cardiomyopathy (may lead to heart failure)
-
Ocular toxicities (uveitis, iritis)
-
-
-
Contraindications: Known hypersensitivity to dabrafenib or its ingredients.
-
Precautions:
-
Regular skin examinations to detect skin malignancies early
-
Monitor for bleeding symptoms; suspend treatment if severe bleeding occurs
-
Advise effective contraception during treatment and for a period after discontinuation due to fetal risk
-
Regular cardiac monitoring (ECG and function tests), especially in patients with cardiovascular risk
-
Periodic ophthalmologic examinations
-
Liver and kidney function tests during treatment
-
Dabrafenib Mesylate Interactions
-
Dabrafenib is primarily used in combination with Trametinib for synergistic effect in treating BRAF V600 mutation-positive cancers.
-
Drug interactions may occur via CYP3A4 metabolic pathway; caution with concomitant medications is advised.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.








Reviews
There are no reviews yet.