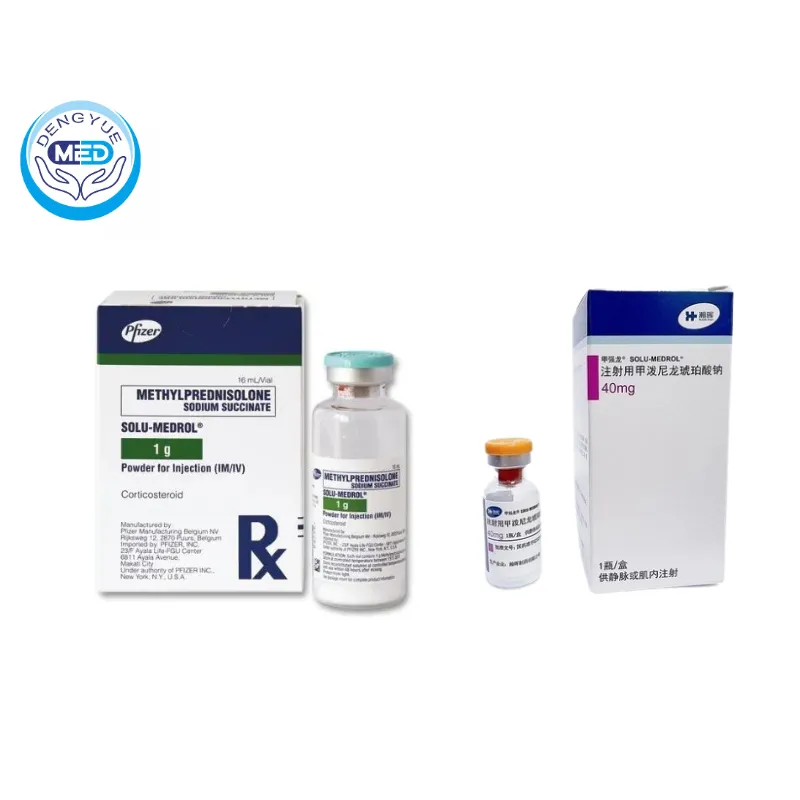
Solu-Medrol (Methylprednisolone Sodium Succinate) – Anti-inflammatory | HongKong DengYue Medicine
- Generic Name/Brand Name: Methylprednisolone Sodium Succinate / Solu-Medrol
- Indications: Inflammation, allergic reactions, and autoimmune or immune-mediated conditions
- Dosage Form: Injectable powder for intravenous (IV) or intramuscular (IM) use
- Specification: 40 mg/125 mg/500 mg/1 g/2 g × 1 vial
MPSS Application Scope
Solu-Medrol is indicated for the treatment of various inflammatory and autoimmune conditions, including:
-
Severe allergic reactions
-
Asthma and chronic obstructive pulmonary disease (COPD) exacerbations
-
Autoimmune disorders such as systemic lupus erythematosus and rheumatoid arthritis
-
Acute inflammatory conditions like cerebral edema and severe skin diseases
-
Immunosuppressive therapy in organ transplantation

solu medrol mpss
MPSS Characteristics
-
Ingredients: Methylprednisolone Sodium Succinate
-
Properties: A white or nearly white, odorless, hygroscopic, amorphous solid
-
Packaging Specification: Available in various strengths: 40 mg, 125 mg, 500 mg, 1 g, and 2 g per vial
-
Storage: Store reconstituted solution at room temperature (15–30°C)
-
Expiry Date: Refer to the expiration date printed on the packaging
-
Executive Standard: Complies with the United States Pharmacopeia (USP) standards for injectable corticosteroids
-
Approval Number: FDA Application Number: NDA 011856
-
Date of Revision: Latest revision as of June 5, 2024
-
Manufacturer: Pfizer Inc.
Guidelines for the Use of MPSS
-
Dosage and Administration:
-
Recommended Dose:
-
Dosage varies based on the specific condition being treated, the severity of the condition, and the patient’s response to therapy.
-
Typical dosing ranges from 40 mg to 1 g per day, administered intravenously or intramuscularly.
-
-
Administration:
-
Reconstitute the powder with the provided diluent or an appropriate sterile diluent.
-
Administer via slow intravenous injection, intravenous infusion, or intramuscular injection, depending on clinical circumstances.
-
-
Missed Dose:
-
If a dose is missed, administer as soon as possible unless it is near the time for the next dose.
-
Do not administer two doses at the same time to make up for a missed dose
-
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Fluid retention
-
Elevated blood pressure
-
Hyperglycemia
-
Mood swings
-
Insomnia
-
-
Serious Adverse Reactions:
-
Increased risk of infections
-
Osteoporosis with long-term use
-
Gastrointestinal bleeding
-
Psychiatric disturbances
-
Cushingoid appearance
-
Adrenal suppression
-
-
-
Contraindications:
-
Systemic fungal infections
-
Known hypersensitivity to methylprednisolone sodium succinate or any component of the formulation
-
Administration of live or live attenuated vaccines during therapy
-
-
Precautions:
-
Use with caution in patients with a history of tuberculosis, peptic ulcer disease, or psychiatric disorders.
-
Gradual withdrawal is recommended after prolonged therapy to prevent adrenal insufficiency.
-
Monitor blood glucose levels in diabetic patients.
-
Avoid abrupt discontinuation to prevent withdrawal symptoms.
-
MPSS Interactions
-
May interact with nonsteroidal anti-inflammatory drugs (NSAIDs), increasing the risk of gastrointestinal bleeding.
-
Concurrent use with diuretics may enhance the risk of hypokalemia.
-
Anticholinesterase agents may have reduced efficacy.
-
Live vaccines may have reduced efficacy and increased risk of adverse reactions.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.






Reviews
There are no reviews yet.