SIRTURO (Bedaquiline) – MDR-TB | HongKong DengYue Medicine
- Generic Name/Brand Name: Bedaquiline/SIRTURO
- Indications: MDR-CM
- Dosage Form: Film-coated tablets
- Specification: 20 mg / 100 mg × 188 tablets
SIRTURO Bedaquiline Application Scope
A diarylquinoline-class antimycobacterial agent specifically indicated for the treatment of multidrug-resistant tuberculosis (MDR-TB) in adult and pediatric patients (≥5 years and ≥15 kg) when an effective treatment regimen cannot otherwise be provided. It must be used in combination with at least 3-4 other active anti-TB drugs to prevent further resistance development.
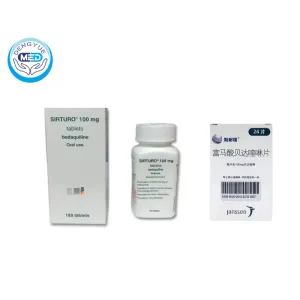
SIRTURO Bedaquiline Characteristics
-
Ingredients: Bedaquiline
-
Properties: 100 mg and 20 mg tablets available
-
Packaging Specification: Typically supplied as 100 mg tablets (188 tablets per box in China)
-
Storage: Store at 25 °C (77 °F); excursions 15–30 °C (59–86 °F) permitted
-
Expiry Date: As labeled by the manufacturer; see bottle
-
Executive Standard: FDA-approved (2012, full approval 2024)
-
Approval Number: NDA 204384 (U.S.)
-
Date of Revision: Last updated in 2024 (FDA full approval)
-
Manufacturer: Manufactured for Janssen Products, LP, Horsham, PA, USA. (Certain lots manufactured by Janssen-Cilag S.p.A., Italy.)
Guidelines for the Use of SIRTURO
-
Dosage and Administration:
-
Adults & Adolescents (≥12 years & ≥30 kg):
-
Weeks 1–2: 400 mg once daily
-
Weeks 3–24: 200 mg three times per week (≥48 hours between doses)
-
-
Children (5–12 years or 15–30 kg):
-
Weeks 1–2: 200 mg once daily
-
Weeks 3–24: 100 mg three times per week
-
-
Missed Dose: If a dose is missed, administer as soon as possible. Resume monthly dosing from the date of the most recently administered dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions (≥5% and >3% higher than placebo):
-
Injection site reactions (19%)
-
Decreased platelet count (12%)
-
Arthralgia (9%)
-
-
Serious Adverse Reactions:
-
Hypersensitivity reactions, including bronchospasm, diffuse erythema, facial swelling, urticaria, chills, and myalgias.
-
-
-
Contraindications:Patients with a history of serious hypersensitivity to olezarsen or any of the excipients in TRYNGOLZA.
-
Precautions:
-
Hypersensitivity Reactions: Monitor patients for signs and symptoms. Discontinue use if reactions occur.
-
Platelet Count Monitoring: Monitor platelet counts during treatment.
-
Pregnancy and Lactation: Advise patients to inform their healthcare provider if they are pregnant, plan to become pregnant, are breastfeeding, or plan to breastfeed.
-
SIRTURO Interactions
- CYP3A4 inducers (↓bedaquiline exposure; reduced efficacy): Avoid—e.g., rifamycins (rifampin, rifapentine, rifabutin), efavirenz, etc.
- CYP3A4 inhibitors (↑exposure; ↑risk of AEs): Use with close monitoring—e.g., ketoconazole, lopinavir/ritonavir, etc.
- Other QT-prolonging drugs: Use cautiously/avoid additive risk (e.g., certain fluoroquinolones, macrolides, clofazimine).
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.

Reviews
There are no reviews yet.