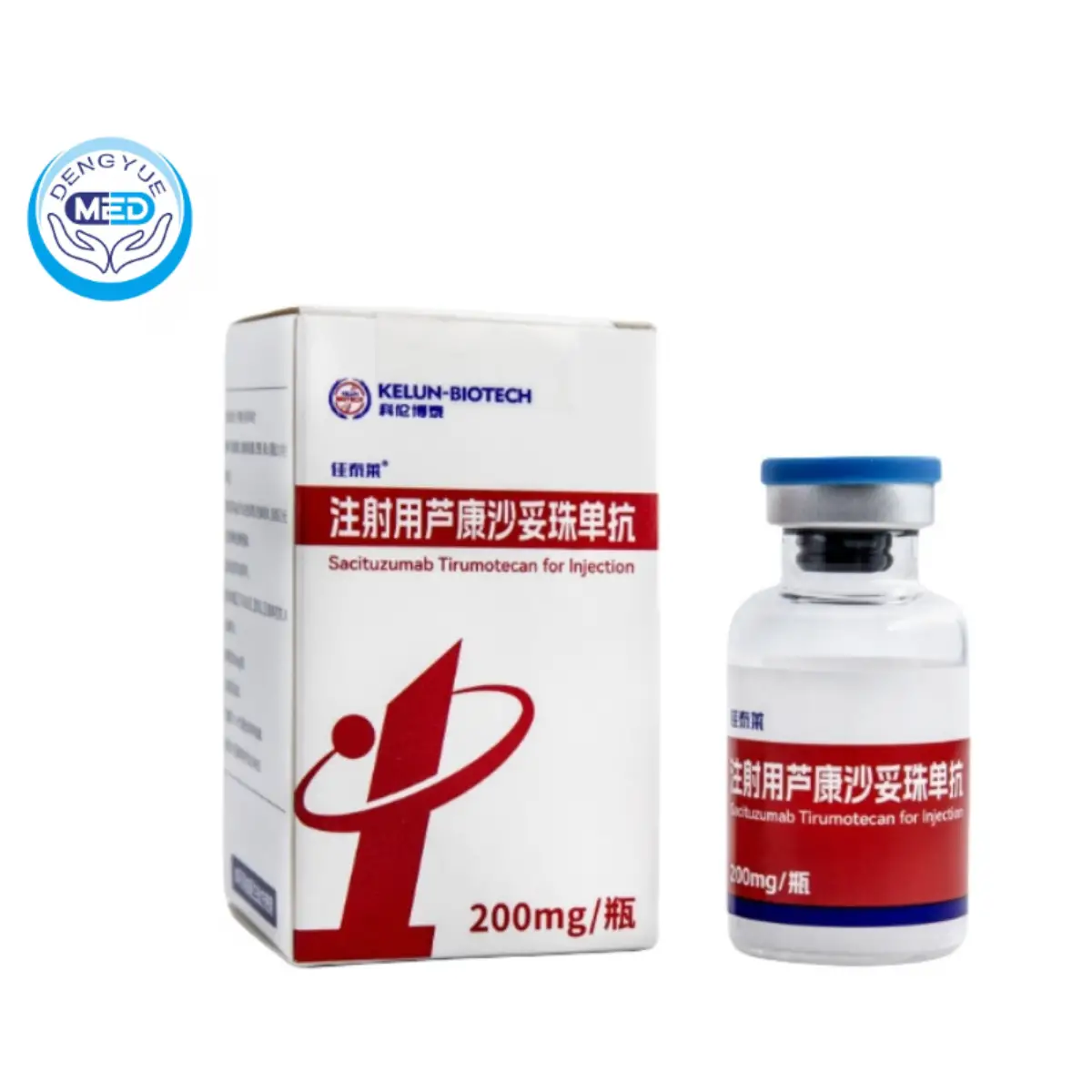
Sacituzumab Tirumotecan For Injection|Triple negative breast cancer |HongKong DengYue Medicine
- Generic Name/Brand Name: Sacituzumab Tirumotecan For Injection(Sacituzumab govitecan)/Gatorade
- Indications: Triple negative breast cancer
- Dosage Form: Solutions
- Specification: 200mg
Sacituzumab Tirumotecan For Injection Application Scope
Sacituzumab tiristomate (brand name: Trodelvy) is primarily used for the treatment of metastatic triple-negative breast cancer (TNBC) after at least two prior therapies. It is also approved for metastatic urothelial cancer in patients who have previously undergone platinum-based chemotherapy and PD-1/PD-L1 inhibitors.
Sacituzumab Tirumotecan For Injection is a targeted therapy combining a monoclonal antibody with a cytotoxic chemotherapy drug, SN-38, which is an active metabolite of irinotecan. This drug selectively targets Trop-2, a cell surface antigen overexpressed in many cancers, including TNBC and urothelial cancer.
Sacituzumab Govitecan Package Insert
- Ingredients: Sacituzumab tiristomate (an anti-Trop-2 monoclonal antibody) conjugated with SN-38.
- Properties:
- Targeted therapy for certain cancers.
- Combines antibody targeting with chemotherapy to deliver a cytotoxic agent directly to tumor cells.
- Approved for use in advanced stages of cancer with limited effective treatments available.
- Specification:
- Concentration: 180 mg/1.8 mL vial.
- Packaging Specification:
- Each box contains one vial.
- Storage:
- Store in a refrigerator at 2°C to 8°C (36°F to 46°F).
- Do not freeze.
- Expiry Date:
- Refer to the packaging for the expiry date.
- Executive Standard:
- Follows FDA and EMA standards for monoclonal antibody therapies.
- Approval Number:
- FDA Approval No: 761157
- EMA Approval No: 1-3214-244
- Date of Revision:
- 2025/02/15
- Manufacturer:
- Gilead Sciences, Inc.
Guidelines For The Use Of Sacituzumab Tirumotecan For Injection
- Dosage and Administration:
- For metastatic TNBC: Administer 10 mg/kg intravenously every 2 weeks. The infusion should be given over 1 hour during the first administration, and can be reduced to 30 minutes for subsequent doses if well tolerated.
- For metastatic urothelial cancer: Administer 10 mg/kg intravenously every 2 weeks after prior platinum-based chemotherapy and PD-1/PD-L1 inhibitor therapy.
- Monitor for infusion-related reactions during and after the infusion. Adjust infusion rate based on tolerance.
- Adverse Reactions:
- Sacituzumab Govitecan Side Effects
- Neutropenia (low white blood cell count)
- Anemia
- Fatigue
- Diarrhea
- Nausea
- Hair loss (alopecia)
- Febrile neutropenia
- Severe:
- Myelosuppression
- Sepsis
- Severe diarrhea
- Infusion-related reactions (e.g., fever, chills, hypotension, difficulty breathing)
Monitoring: Regular blood tests for complete blood count (CBC) to monitor for neutropenia, anemia, and thrombocytopenia. Patients should also be monitored for signs of gastrointestinal toxicities.
- Sacituzumab Govitecan Side Effects
Sacituzumab Tirumotecan For Injection Medication Limitations
- Contraindications:
- Hypersensitivity to sacituzumab tiristomate or any of the excipients.
- Severe myelosuppression or active infections.
- Precautions:
- Use with caution in patients with a history of gastrointestinal disorders or active diarrhea.
- Requires monitoring for neutropenia and diarrhea, as both can be severe.
- Caution should be exercised in pregnant women due to potential risks to the fetus, as the drug is categorized as Pregnancy Category D.
Sacituzumab Tirumotecan For Injection Drug Interactions
- Known Drug Interactions:
- CYP3A4 inhibitors and inducers: As Sacituzumab Tirumotecan is metabolized by the liver enzymes, using strong CYP3A4 inhibitors or inducers may alter its pharmacokinetics and require dose adjustments.
- Live vaccines: Avoid live vaccines during treatment due to potential immune system suppression.
- Other Considerations:
- Caution is advised when combining Sacituzumab Tirumotecan with other myelosuppressive agents or other immunosuppressive drugs, as it can increase the risk of severe infections and adverse effects.
Please note that this information is based on available data and may be subject to change as more research and clinical experience emerge. Always consult with healthcare professionals before beginning treatment.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine. It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.





Reviews
There are no reviews yet.