Rystiggo (Rozanolixizumab) – gMG | HongKong DengYue Medicine
- Generic Name/Brand Name: Rozanolixizumab/Rystiggo
- Indications: gMG & AChR & MuSK
- Dosage Form: Solution for injection
- Specification: 280 mg × 1 vial
Rozanolixizumab Application Scope
Rozanolixizumab is indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) or anti-muscle-specific tyrosine kinase (MuSK) antibody positive.
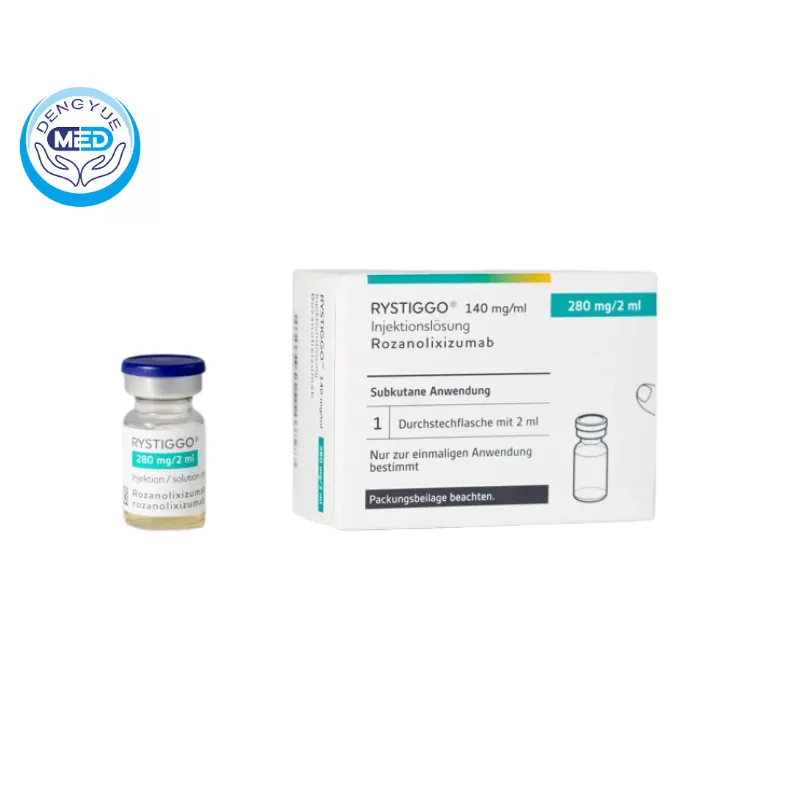
Rozanolixizumab Characteristics
-
Ingredients: Rozanolixizumab (a humanized IgG4 monoclonal antibody targeting the neonatal Fc receptor, FcRn)
-
Properties: A sterile, preservative-free, colorless to slightly yellowish solution for subcutaneous injection.
-
Packaging Specification: 280 mg/2 mL (140 mg/mL) in a single-dose glass vial; 1 vial per carton.
-
Storage: Store refrigerated (typically to ( to )). Do not freeze. Protect from light.
-
Expiry Date: Not available in general public information (consult product packaging/vial).
-
Executive Standard: Not available in general public information (consult prescribing information for specific standard).
-
Approval Number: To be provided according to local regulatory authority records.
-
Date of Revision: Not available in general public information (consult current prescribing information).
-
Manufacturer: UCB, Inc.
Guidelines for the Use of Rozanolixizumab
-
Dosage and Administration:
-
Recommended Dose: Based on body weight, administered once weekly for 6 weeks: – Less than 50 kg: 420 mg SC infusion (3 mL) – 50 kg to less than 100 kg: 560 mg SC infusion (4 mL) – 100 kg and above: 840 mg SC infusion (6 mL)
-
Administration: For subcutaneous infusion only, using an infusion pump. – Should be prepared and infused by a healthcare provider. – Infusion rate: up to 20 mL/hour. – Preferred sites: lower right or lower left part of the abdomen, below the belly button. Avoid areas that are tender, erythematous, or indurated.
-
Missed Dose: Specific instructions for a missed dose should be provided by the healthcare provider. Consult the full prescribing information.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Headache
-
Nausea
-
Diarrhea
-
Fever
-
Injection site reactions
-
-
Serious Adverse Reactions:
-
Hypersensitivity or allergic reactions
-
Infections due to decreased IgG levels
-
-
-
Contraindications:
-
Known hypersensitivity to rozanolixizumab or any component of the formulation
-
Active or severe infections
-
-
Precautions:
-
Infections: Delay in active infection; monitor for signs. Avoid live vaccines during treatment. Vaccinate per guidelines before new cycle.
-
Aseptic meningitis: Monitor for symptoms (e.g., severe headache, neck stiffness); initiate diagnostic workup if suspected.
-
Hypersensitivity: Monitor during/after infusion; discontinue if occurs.
-
Pregnancy: May cause fetal harm based on animal data; use effective contraception. No data in lactation or pediatrics.
-
Rozanolixizumab Interactions
-
May reduce systemic exposure and effectiveness of medications binding to FcRn (e.g., immunoglobulins, Fc-containing monoclonal antibodies). Monitor closely; consider alternatives if long-term concomitant use is essential. No clinical drug interaction studies performed; not metabolized by CYP450 enzymes.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.









Reviews
There are no reviews yet.