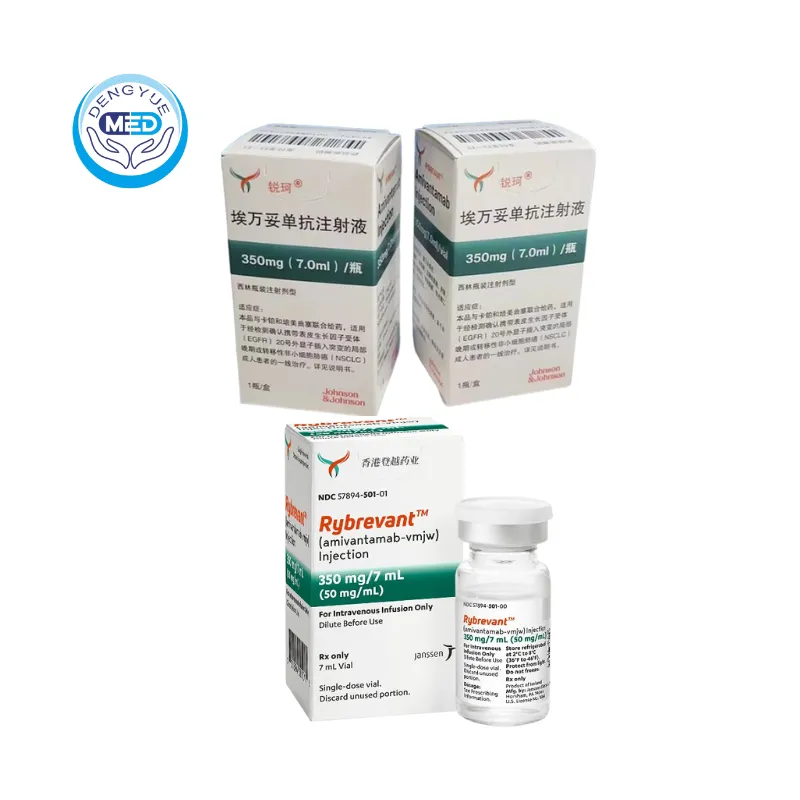
Rybrevant (Amivantamab) – NSCLC | HongKong DengYue Medicine
- Generic Name/Brand Name: Amivantamab / Rybrevant®
- Indications: NSCLC
- Dosage Form: Injection
- Specification: 350 mg/7 mL × 1 vial/box
Amivantamab Application Scope
-
EGFR Exon 19 del or Exon 21 L858R (first-line): In combination with lazertinib for locally advanced or metastatic NSCLC.
-
EGFR Exon 19 del/L858R (post-EGFR TKI): In combination with carboplatin + pemetrexed.
-
EGFR Exon20 insertion (first-line): In combination with carboplatin + pemetrexed.
-
EGFR Exon20 insertion (post-platinum): Single-agent.

rybrevant amivantamab
Amivantamab Characteristics
-
Ingredients: Each single-dose vial contains amivantamab-vmjw 350 mg/7 mL (50 mg/mL) with excipients: EDTA disodium salt dihydrate, L-histidine, L-histidine HCl monohydrate, L-methionine, polysorbate 80, sucrose, water for injection.
-
Properties: Bispecific human IgG1 monoclonal antibody targeting EGFR and MET; sterile, preservative-free, colorless to pale yellow solution for IV infusion.
-
Packaging Specification: Single-dose vial 350 mg/7 mL (50 mg/mL); one vial per carton
-
Storage: Refrigerate 2–8 °C (36–46 °F) in original carton; protect from light; do not freeze.
-
Expiry Date: As printed on the vial/carton; do not use beyond the labeled expiration date.
-
Executive Standard: US FDA Prescribing Information (USPI), revised 02/2025.
-
Approval Number: US BLA 761210.
-
Date of Revision: February 2025 (02/2025).
-
Manufacturer: Janssen Biotech, Inc. (Johnson & Johnson Innovative Medicine).
Guidelines for the Use of Rybrevant
-
Dosage and Administration:
-
Recommended Dose: Weight-based as above; premedications required prior to each infusion; use peripheral line for Week 1–2 to reduce IRR risk.
-
Administration: Dilute in 0.9% NaCl; split the initial dose over Day 1 and Day 2 (Week 1); follow label-specified infusion rates; monitor closely for infusion-related reactions (IRR), especially first infusion.
-
Missed Dose: Administer the missed dose as soon as possible and adjust to maintain the q2-week (lazertinib/single-agent) or q3-week (chemo combo) schedule thereafter.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
With lazertinib: rash, nail toxicity, IRR, musculoskeletal pain, stomatitis, edema, VTE, paresthesia, fatigue, diarrhea/constipation, bleeding, dry skin, ↓appetite, pruritus, nausea, ocular effects.
-
With carboplatin+pemetrexed: rash, nail toxicity, IRR, fatigue, nausea, stomatitis, constipation, edema, ↓appetite, musculoskeletal pain, vomiting.
-
Single-agent: rash, IRR, paronychia, musculoskeletal pain, dyspnea, nausea, fatigue, edema, stomatitis, cough, constipation, vomiting.
-
-
Serious Adverse Reactions: ILD/pneumonitis, severe IRR (incl. anaphylaxis), VTE (esp. with lazertinib), severe dermatologic reactions (incl. TEN), ocular toxicity, and embryo-fetal toxicity. Manage with withhold/reduce/discontinue per severity.
-
-
Contraindications: None listed.
-
Precautions:
-
IRR: most occur on first infusion; premedicate and use peripheral line early.
-
ILD/pneumonitis: withhold if suspected; discontinue if confirmed.
-
VTE with lazertinib: prophylactic anticoagulation recommended for first 4 months; monitor/manage.
-
Dermatologic/Ocular toxicity: institute prophylaxis (emollients; consider oral antibiotics), UV protection; prompt ophthalmology referral for symptoms.
-
Embryo-fetal toxicity: use effective contraception; avoid breastfeeding.
-
Amivantamab Interactions
-
Metabolism/PK: As a monoclonal antibody, not CYP-metabolized; clinically meaningful CYP-mediated DDIs are unlikely.
-
Regimen-specific risks: With lazertinib, increased VTE risk → use anticoagulant prophylaxis initially. With platinum/pemetrexed, anticipate additive myelosuppression and GI toxicity.
-
Vaccines: Avoid live vaccines during and shortly after therapy; inactivated vaccines may be used as appropriate.
-
QT/CYP effects on co-meds: No evidence that amivantamab prolongs QT or induces/inhibits CYP enzymes.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.








Reviews
There are no reviews yet.