ONIVYDE (Irinotecan Liposome) – Pancreatic Cancer | HongKong DengYue Medicine
- Generic Name/Brand Name: Irinotecan Liposome / ONIVYDE®
- Indications: Pancreatic Cancer
- Dosage Form: Injection
- Specification: 43 mg × 1 vial/box
Irinotecan Liposome Application Scope
Indication: Metastatic pancreatic adenocarcinoma — in combination with fluorouracil (5-FU) and leucovorin for patients whose disease progressed following gemcitabine-based therapy; also indicated in specific first-line combinations for metastatic disease.
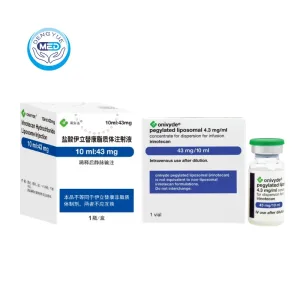
Irinotecan Liposome Characteristics
-
Ingredients: Irinotecan liposome (irinotecan encapsulated in pegylated/unilamellar liposomes).
-
Properties: Liposomal formulation of irinotecan that prolongs circulation time and alters pharmacokinetics compared with non-liposomal irinotecan; inhibits topoisomerase I, causing DNA damage in replicating tumor cells.
-
Packaging Specification: 43 mg × 10 mL per vial (single-dose vial).
-
Storage: Refrigerate at 2 °C – 8 °C; protect from light; do not freeze.
-
Expiry Date: See package label.
-
Executive Standard: Product-specific pharmaceutical quality standard (refer to label).
-
Approval Number: NDA 207793 (U.S. regulatory filing).
-
Date of Revision: Refer to the latest package insert.
-
Manufacturer: Ipsen Biopharmaceuticals, Inc. (commercialized as ONIVYDE®).
Guidelines for the Use of ONIVYDE
-
Dosage and Administration:
-
Recommended Dose: 70 mg/m² irinotecan liposome (as irinotecan free base) administered as an intravenous infusion over 90 minutes every 2 weeks, given before leucovorin and 5-fluorouracil.
-
Administration: Intravenous infusion after appropriate dilution and preparation; administer before leucovorin and 5-FU per combination regimen instructions.
-
Missed Dose: Reschedule as soon as possible per treating physician; do not double doses.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Diarrhea, nausea, vomiting, fatigue, abdominal pain, decreased appetite, neutropenia, anemia, thrombocytopenia, fever, mucositis.
-
Serious Adverse Reactions: Severe neutropenia (including neutropenic sepsis), severe diarrhea (which can be life-threatening), sepsis, severe hematologic toxicity, and severe infections. Boxed warnings apply for severe neutropenia and severe diarrhea.
-
-
Contraindications: Known hypersensitivity to irinotecan, irinotecan liposome components, or excipients. Do not substitute ONIVYDE for other irinotecan formulations (not interchangeable).
-
Precautions:
-
Monitor complete blood counts before and during therapy, and manage neutropenia according to dosing guidelines.
-
Monitor for and promptly treat diarrhea; provide prophylactic/therapeutic anti-diarrheal measures as indicated.
-
Assess hepatic function before and during treatment; dosing modification may be required for hepatic impairment.
-
Use caution in patients with active infections or significant comorbidities.
-
Genetic testing for UGT1A1*28 may guide starting dose selection.
-
Irinotecan Liposome Interactions
-
Avoid substituting with non-liposomal irinotecan — formulations are not bioequivalent.
-
UGT1A1 genotype affects metabolism and toxicity risk (UGT1A1*28 homozygotes at increased risk of neutropenia → consider lower starting dose).
-
Concomitant use with strong inhibitors or inducers of drug-metabolizing enzymes may alter exposure to irinotecan metabolites; monitor closely.
-
Caution when combined with other myelosuppressive, hepatotoxic, or diarrhea-causing agents.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.
Related products
-

Lentinus Edodes Mycelia Polysacharide Tablets|Malignant Tumour
-

Elranatamab|Multiple Myeloma|HongKong DengYue Medicine
-

Gemcitabine Hydrochloride Injection | Pancreatic Cancer
-

Cidofovir Injection | Anti CMV,AIDS | DengYueMed
-

Bleomycin A5 Hydrochloride For Injection|Tongue Cancer
-

Etoposide Soft Capsules | Ovarian Cancer | China Medicine Wholesaler
-

Prednisone Acetate Tablets|Immune Inflammatory Diseases
-

Compound Cyclophosphamide Tablets | Ovarian Cancer | DengYueMed






Reviews
There are no reviews yet.