Ojemda (Tovorafenib) – Pediatric Low-Grade Glioma | HongKong DengYue Medicine
- Generic Name/Brand Name: Tovorafenib / Ojemda
- Indications: Pediatric Low-Grade Glioma
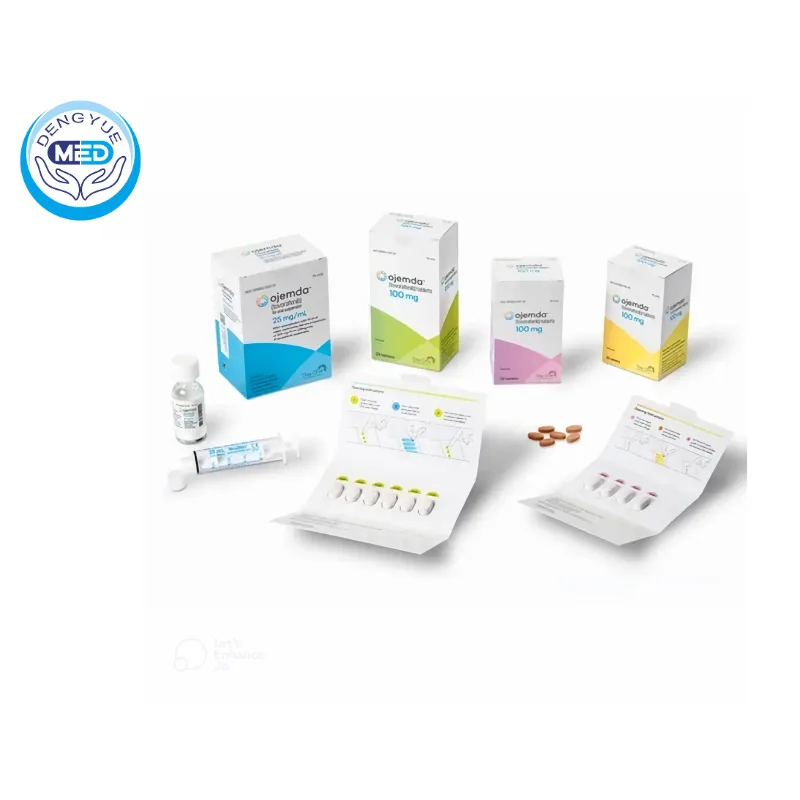
- Dosage Form: Film-coated tablets and powder for oral suspension.
- Specification: Pediatric Low-Grade Glioma
Ojemda Application Scope
Ojemda (tovorafenib) is designed to target the MAPK signaling pathway by inhibiting RAF kinases, including wild-type and certain mutant forms of A-Raf, B-Raf, and C-Raf.
This inhibition disrupts tumor cell growth and proliferation, particularly in gliomas harboring BRAF alterations.
Characteristics
-
Ingredients: Tovorafenib
-
Properties: Tovorafenib is a kinase inhibitor used in the treatment of glioma, specifically targeting BRAF alterations.
-
Packaging Specification: Available as film-coated tablets (100 mg) and as a powder for oral suspension (25 mg/mL after reconstitution).
-
Storage:
- Tablets:
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F).
- Dispense in original packaging; do not remove tablets from blisters until immediately before use.
- Oral Suspension:
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F).
- Use immediately after reconstitution; discard any unused portion.
- Tablets:
-
Expiry Date: Refer to the packaging for the expiration date.
-
Executive Standard: Not specified in the available information.
-
Approval Number: Not specified in the available information.
-
Date of Revision: Not specified in the available information.
-
Manufacturer: Day One Biopharmaceuticals, Inc.
Guidelines for the Use of Ojemda
-
Dosage and Administration:
-
Administration: Take Ojemda once weekly, with or without food.
-
Tablets: Swallow whole with water; do not chew, cut, or crush.
-
Oral Suspension:
-
Reconstitute each bottle with 14 mL of room temperature water to achieve a concentration of 25 mg/mL.
-
Administer immediately after preparation using the supplied oral dosing syringe or feeding tube.
-
Discard if not used within 15 minutes.
-
-
Missed Dose:
-
If missed by ≤3 days: Take as soon as possible and continue with the regular schedule.
-
If missed by >3 days: Skip the missed dose and resume on the next scheduled day.
-
-
Vomiting: If vomiting occurs immediately after dosing, repeat the dose.
-
-
Adverse Reactions:
-
Common (≥30%): Rash, hair color changes, fatigue, viral infections, vomiting, headache, hemorrhage, pyrexia, dry skin, constipation, nausea, dermatitis acneiform, upper respiratory tract infection.
-
Serious Adverse Reactions: Hemorrhage, skin toxicity including photosensitivity, hepatotoxicity, and effects on growth.
-
-
Contraindications: Specific contraindications are not detailed in the available information.
-
Precautions:
-
Pediatric Use: Safety and effectiveness established in patients aged 6 months and older with relapsed or refractory pediatric low-grade glioma harboring a BRAF fusion or rearrangement, or BRAF V600 mutation. Monitor growth routinely during treatment.
-
Renal Impairment: No dose adjustment recommended for mild-to-moderate impairment; not studied in severe impairment.
-
Hepatic Impairment: No dose adjustment recommended for mild impairment; not studied in moderate to severe impairment.
-
Interactions
-
Drug Interactions: Specific drug interactions are not detailed in the available information.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.









Reviews
There are no reviews yet.