NUZYRA (Omadacycline) – Antibiotics | HongKong DengYue Medicine
- Generic Name/Brand Name: Omadacycline/NUZYRA
- Indications: CABP, ABSSSI
- Dosage Form: Oral Tablets, Injection
- Specification: 0.1g x 1 vial
NUZYRA Omadacycline Application Scope
An aminomethylcycline antibiotic used to treat community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI) in adults caused by susceptible pathogens.
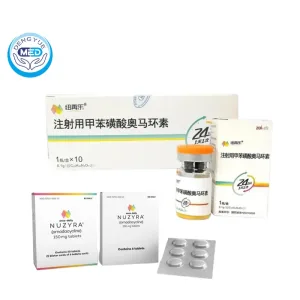
NUZYRA Omadacycline Characteristics
-
Ingredients: Omadacycline (as omadacycline tosylate)
-
Properties: Broad-spectrum tetracycline-class antibiotic; inhibits bacterial protein synthesis; effective against common Gram-positive, Gram-negative, and atypical pathogens.
-
Packaging Specification:
-
Injection: 100 mg single-dose lyophilized vial
-
Tablets: 150 mg film-coated tablets (bottles of 30)
-
- Storage: Store at 20–25 °C (USP room temperature); do NOT freeze
- Expiry Date: Refer to vial or bottle label (typically up to 30 months when stored correctly)
- Executive Standard: Compliant with FDA and EMA requirements; meets pharmaceutical and USP standards for antibiotics
- Approval Number:
FDA-approved Oct 2 2018 (NDA 209816 and 209817) for tablet and injection formulations - Date of Revision: Update
- Manufacturer: Paratek Pharmaceuticals, Inc. (USA)
Guidelines for the Use of NUZYRA
-
Dosage and Administration:
-
Day 1: 200 mg IV over 60 min or 100 mg IV over 30 min twice
-
Maintenance: 100 mg IV daily or 300 mg orally daily for 7–14 days
-
-
Adverse Reactions:
- Nausea, vomiting, infusion‑site reactions, elevated liver enzymes, hypertension, headache, diarrhea, insomnia, constipation
-
Serious Adverse Reactions:
-
C. difficile-associated diarrhea, photosensitivity, tooth discoloration, bone growth inhibition (in children/pregnancy), allergic/anaphylactic reactions
-
-
Contraindications: Known hypersensitivity to omadacycline, tetracycline-class antibiotics, or excipients
-
Precautions:
-
-
Monitor for C. difficile-associated diarrhea and hypersensitivity
-
Avoid use in pregnant women and children <8 years due to effects on teeth/bone
-
Monitor elderly patients due to observed mortality imbalance in CABP trial (>65 years)
-
-
NUZYRA Interactions
-
May require adjustments for anticoagulants
-
Oral absorption reduced by antacids with aluminum, calcium, magnesium, iron, and bismuth
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.






Reviews
There are no reviews yet.