Mekinist (Trametinib) – NSCLC | DengYue Medicine
- Generic Name/Brand Name: Trametinib/Mekinist
- Indications: NSCLC (Lung Cancer)
- Dosage Form: Film-coated Tablets
- Specification: 2 mg x 30 tablets
Mekinist Application Scope
Mekinist is an oral MEK1/MEK2 inhibitor used in treating BRAF V600-mutant cancers.
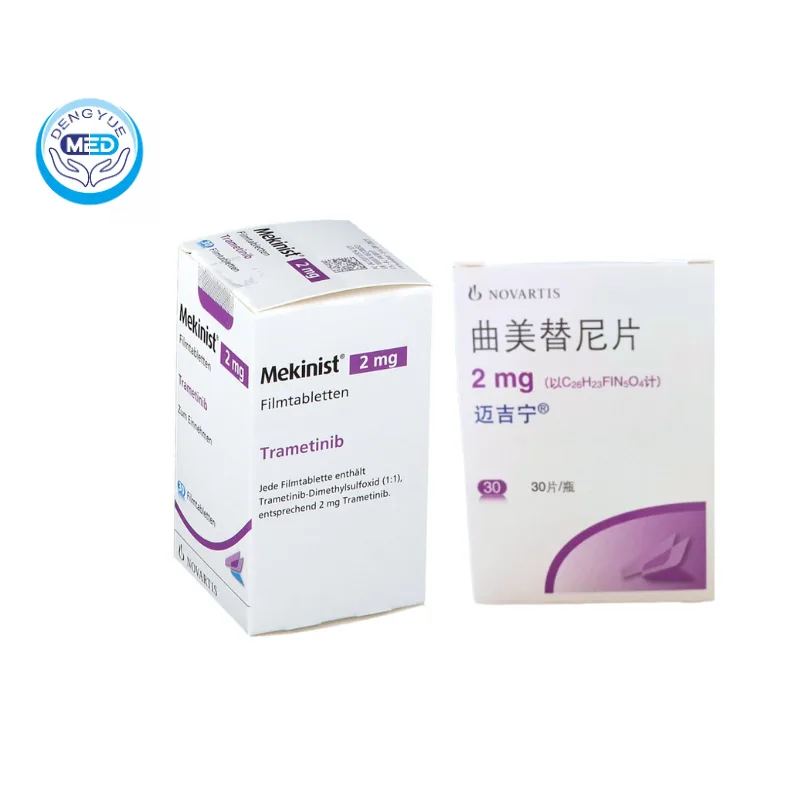
Characteristics
-
Ingredients: Trametinib
-
Properties:
-
Oral kinase inhibitor against MEK1/2, interrupting MAPK signaling
-
Available as film-coated tablets (0.5 mg yellow oval, 2 mg pink round)
-
-
Packaging Specification: Tablets supplied in bottles of 7 or 30 with screw-cap closures
-
Storage: Store in original container at controlled room temperature, protect from moisture
-
Expiry Date: Refer to bottle label; typically two years from manufacture
-
Executive Standard: Conforms to FDA, EMA, and Health Canada prescribing information
-
Approval Number: US NDA 204114; Health Canada authorization under the same references
-
Date of Revision: US labeling updated January–March 2025
-
Manufacturer: Novartis Europharm Limited (Ireland); tablets produced in Slovenia (Lek)
Guidelines for the Use of Mekinist
-
Dosage and Administration:
-
Adults: 2 mg orally once daily, at least 1 hour before or 2 hours after food
-
Pediatric: weight-based dosing (e.g., 1 mg for 26–37 kg; 2 mg for ≥51 kg)
-
Combination:
-
used with dabrafenib 150 mg twice daily for BRAF-mutant melanoma
-
NSCLC, thyroid cancer, glioma
-
-
Dose reduction steps: 1.5 mg → 1 mg → hold/discontinue if not tolerated
-
-
Adverse Reactions:
-
Common:
-
rash, diarrhea, fatigue, peripheral edema, nausea, acneiform dermatitis
-
-
With dabrafenib:
-
fever, chills, headache, arthralgia, hemorrhage, cardiomyopathy, ocular issues
-
-
-
Contraindications:
-
Hypersensitivity to trametinib or excipients
-
No other absolute contraindications are listed
-
-
Precautions:
-
Monitor for new primary malignancies, bleeding
-
DVT/PE, cardiomyopathy, retinal vein occlusion, interstitial lung disease
-
Mekinist Interactions
-
Drug Interactions:
-
Avoid strong CYP3A4 inducers or inhibitors
-
Space dosing around such medications
-
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.







Reviews
There are no reviews yet.