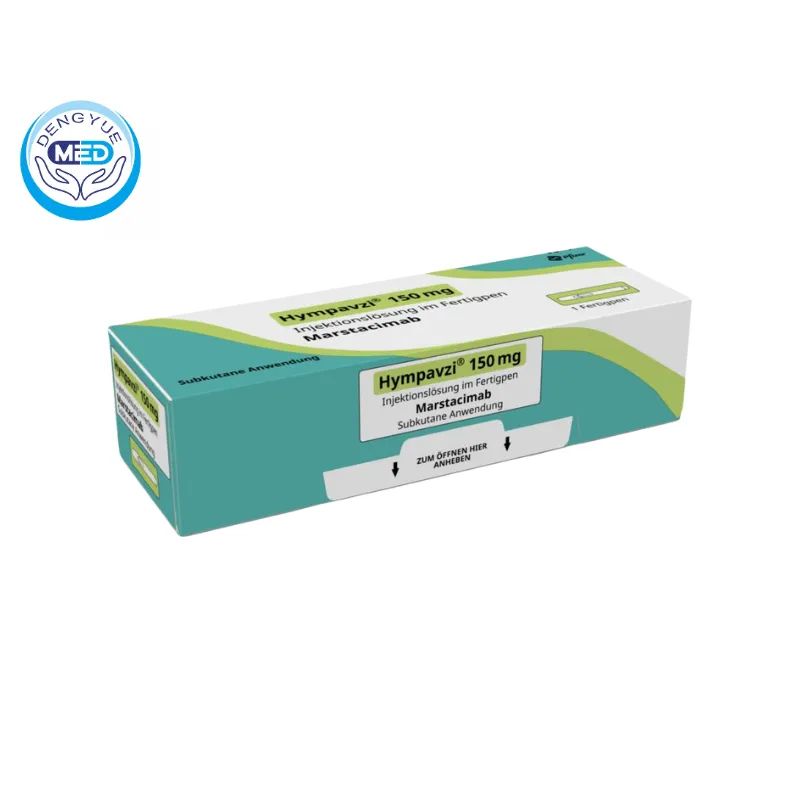
Marstacimab-Hncq|Hemophilia|HongKong DengYue Medicine
- Generic Name/Brand Name: Marstacimab-hncq / HYMPAVZI
- Indications: Hemophilia
- Dosage Form: Injection
- Specification: 150 mg/mL
Marstacimab-Hncq Application Scope
HYMPAVZI is indicated for routine prophylaxis to prevent or reduce the frequency of bleeding episodes in adults and pediatric patients aged 12 years and older with:
-
Hemophilia A (congenital factor VIII deficiency) without factor VIII inhibitors
-
Hemophilia B (congenital factor IX deficiency) without factor IX inhibitors
It is the first once-weekly subcutaneous prophylactic treatment for eligible individuals with hemophilia B and the first to be administered via a pre-filled pen or syringe for eligible individuals with hemophilia A or B in the U.S.
Marstacimab-Hncq Characteristics
-
Ingredients: Each 1 mL contains 150 mg of marstacimab-hncq and inactive ingredients including edetate disodium, histidine, L-histidine monohydrochloride, polysorbate 80, sucrose, in Water for Injection, USP.
-
Properties: Marstacimab-hncq is a human monoclonal IgG1 antibody that binds to the Kunitz domain 2 (K2) of tissue factor pathway inhibitor (TFPI), neutralizing its activity and enhancing coagulation.
-
Packaging Specification: Available as a sterile, preservative-free solution in a single-dose prefilled syringe or pen for subcutaneous administration.
-
Storage: Store refrigerated at 2°C to 8°C (36°F to 46°F). Do not freeze. Protect from light.
-
Expiry Date: Refer to the expiration date printed on the syringe or pen label. Do not use if the expiration date has passed.
-
Executive Standard: Approved by the U.S. Food and Drug Administration (FDA)
-
Approval Number: Biologics License Application (BLA) 761369
-
Date of Revision: October 11, 2024
-
Manufacturer: Pfizer Inc.
Guidelines for the Use of Marstacimab-Hncq
Dosage and Administration:
For patients aged 12 years and older:
-
Loading Dose: 300 mg subcutaneously (two 150 mg injections)
-
Maintenance Dose: 150 mg subcutaneously once weekly, starting one week after the loading dose, on the same day each week at any time of day.
Adverse Reactions:
Common adverse reactions (≥3%) include:
-
Injection site reactions (pain, itching, swelling, bruising, hardening, or redness)
-
Headache
-
Pruritus (itching)
Serious adverse reactions are rare but may include hypersensitivity reactions such as urticaria and pruritus.
Contraindications:
Known hypersensitivity to marstacimab-hncq or any of its components.
Precautions:
-
Monitor for signs of hypersensitivity reactions; discontinue use if severe reactions occur.
-
Not recommended for use in patients with inhibitors to factor VIII or IX.
-
The safety and efficacy in patients under 12 years of age have not been established.
Marstacimab-Hncq Interactions
Drug Interactions:
No clinically significant differences in standard measures of coagulation, including activated partial thromboplastin time (aPTT) and prothrombin time (PT), were observed following marstacimab-hncq therapy.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.








Reviews
There are no reviews yet.