Margenza (Margetuximab) – HER2 | HongKong DengYue Medicine
- Generic Name/Brand Name: Margetuximab-cmkb/MARGENZA
- Indications: HER2-positive metastatic breast cancer
- Dosage Form: Solution for intravenous infusion
- Specification: 250 mg × 4 vials
Margetuximab Application Scope
Indicated, in combination with chemotherapy, for the treatment of adult patients with metastatic HER2-positive breast cancer who have received two or more prior anti-HER2 regimens, at least one of which was for metastatic disease.
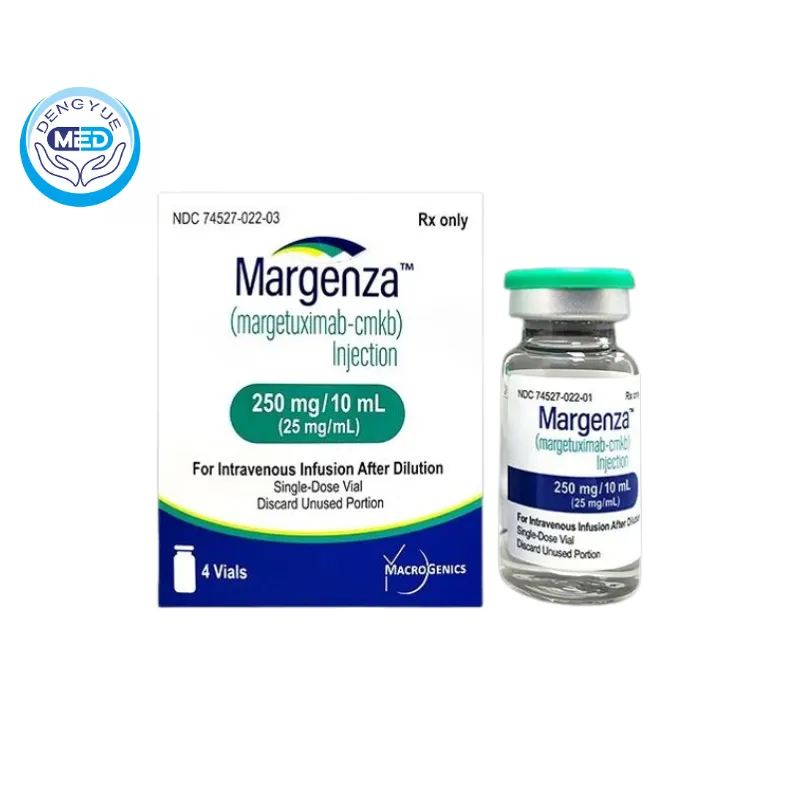
Margetuximab Characteristics
-
Ingredients: Margetuximab-cmkb
-
Properties: Human/mouse chimeric Fc-engineered IgG1 kappa monoclonal antibody; clear to slightly opalescent, colorless to pale yellow or pale brown solution.
-
Packaging Specification: Vial containing 250 mg/10 mL of Margetuximab-cmkb concentrate for solution for infusion.
-
Storage: Refrigerate at to ( to ) in the original carton to protect from light.
-
Expiry Date: Not universally available; refer to the manufacturer’s labeling on the package for the specific product’s expiration date.
-
Executive Standard: According to the manufacturer’s internal quality standard and relevant regulatory guidelines.
-
Approval Number: Refer to the approved local regulatory registration number.
-
Date of Revision: Refer to the latest version of the manufacturer’s Full Prescribing Information.
-
Manufacturer: MacroGenics, Inc. (Marketed under the brand name Margenza).
Guidelines for the Use of Margetuximab
-
Dosage and Administration:
-
Recommended Dose: 15 mg/kg intravenously every 3 weeks, in combination with chemotherapy.
-
Administration: Infuse over approximately 120 minutes for the first dose, and 30 minutes for subsequent doses if tolerated. Do not administer as an IV push or bolus.
-
Missed Dose: If a scheduled dose is missed, administer it as soon as possible; do not double the next dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Fatigue, nausea, diarrhea, infusion-related reactions, vomiting, headache, and fever.
-
Serious Adverse Reactions: Infusion-related reactions, cardiotoxicity, and severe hypersensitivity reactions.
-
-
Contraindications: No specific contraindications are listed in the FDA Prescribing Information, but the Boxed Warnings regarding Left Ventricular Dysfunction and Embryo-Fetal Toxicity are critical considerations.
-
Precautions:
-
Monitor cardiac function before and during treatment.
-
Observe patients for infusion-related reactions.
-
Use caution in patients with a history of congestive heart failure or cardiac dysfunction.
-
Margetuximab Interactions
- No specific drug-drug interaction studies have been conducted. Avoid or use alternate drugs when considering a combination with other immunosuppressive therapies due to the potential for additive immune system effects and risk of infection.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.






Reviews
There are no reviews yet.