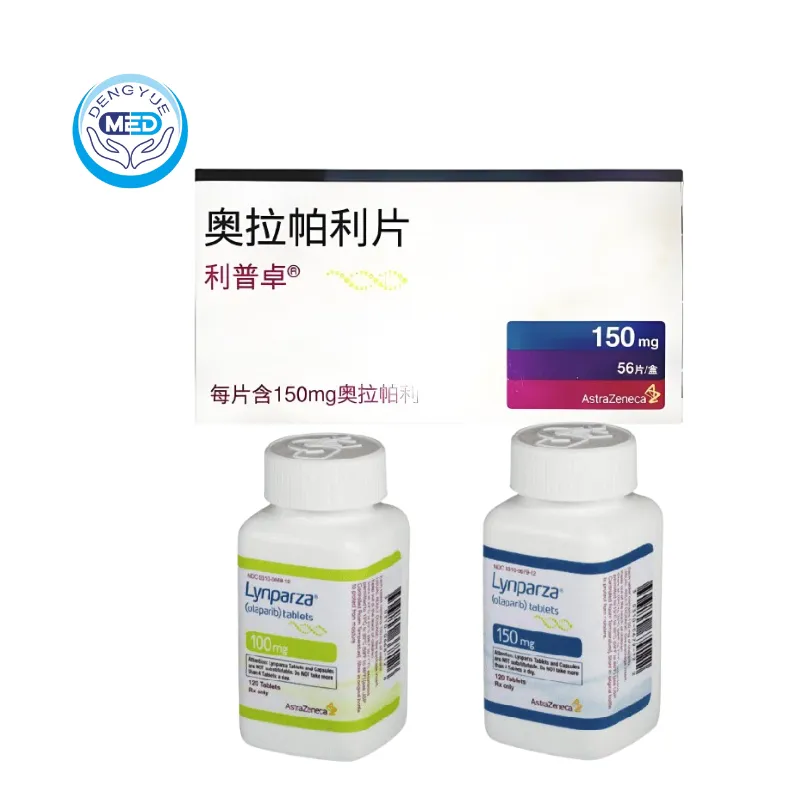
Lynparza (Olaparib) – Ovarian Cancer | HongKong DengYue Medicine
- Generic Name/Brand Name: Olaparib / Lynparza®
- Indications: BRCA-mutated ovarian, breast, pancreatic, and prostate cancers
- Dosage Form: Film-coated tablets
- Specification: 150 mg × 60 tablets per box
Olaparib Application Scope
• Maintenance treatment of adult patients with BRCA-mutated ovarian cancer, fallopian tube cancer, or primary peritoneal cancer
• Treatment of HER2-negative, BRCA-mutated metastatic breast cancer
• BRCA-mutated metastatic pancreatic cancer
• BRCA-mutated, castration-resistant prostate cancer

Olaparib Characteristics
-
Ingredients: Olaparib
-
Properties: Film-coated tablets, off-white to yellowish-white, oval-shaped
-
Packaging Specification: 100 mg or 150 mg per tablet, available in bottles of 60 tablets
-
Storage: Store at room temperature below 30°C. Keep in the original container to protect from moisture.
-
Expiry Date: Refer to outer packaging (typically 24 months from manufacture)
-
Executive Standard: In compliance with FDA and EMA regulatory standards
-
Approval Number: Example (U.S.): NDA 208558 (150 mg tablet)
-
Date of Revision: August 2025 (fill with most recent date if available)
-
Manufacturer: AstraZeneca UK Limited
Guidelines for the Use of Lynparza
-
Dosage and Administration:
-
Recommended Dose: 300 mg (two 150 mg tablets) orally twice daily
-
Administration: Oral route; consistent timing each day is advised
-
Missed Dose: If a dose is missed, take the next dose at the usual scheduled time. Do not take extra tablets to make up for a missed dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions (≥5% and >3% higher than placebo):
• Nausea
• Fatigue (including asthenia)
• Anemia
• Vomiting
• Diarrhea
• Decreased appetite -
Serious Adverse Reactions:
• Myelodysplastic syndrome/Acute myeloid leukemia (MDS/AML)
• Pneumonitis
• Severe anemia and other hematologic toxicities
-
-
Contraindications:Patients with known hypersensitivity to olaparib or any component of the formulation
-
Precautions:
-
-
Myelosuppression:
Monitor blood counts monthly during treatment -
MDS/AML Risk:
Discontinue treatment if confirmed -
Pulmonary Toxicity:
Monitor for new or worsening respiratory symptoms -
Pregnancy and Lactation:
Avoid use during pregnancy; advise effective contraception. Discontinue breastfeeding during treatment and for 1 month after last dose
-
-
Olaparib Interactions
-
CYP3A Inhibitors/Inducers:
• Avoid strong or moderate CYP3A inhibitors (e.g., ketoconazole)
• Avoid CYP3A inducers (e.g., rifampin, carbamazepine)
• Adjust dose if coadministration unavoidable -
Other considerations:
• Use caution with drugs causing myelosuppression
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.








Reviews
There are no reviews yet.