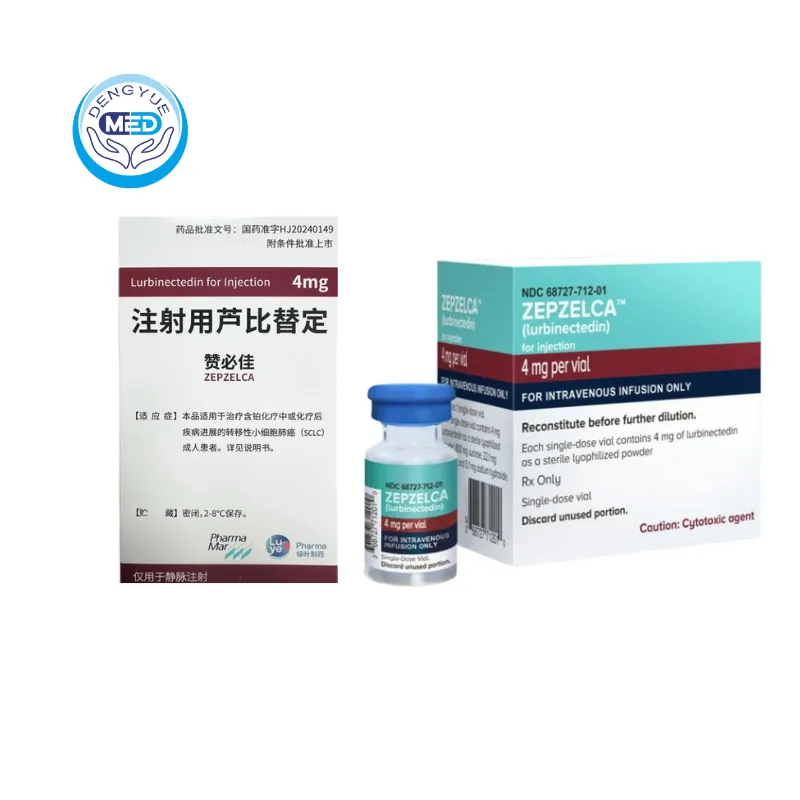
Zepzalca (Lurbinectedin) – NSCLC|HongKong DengYue Medicine
- Generic Name/Brand Name: Lurbinectedin / Zepzelca®
- Indications: NSCLC
- Dosage Form: Injection
- Specification: 4mg × 1 vial/box
Lurbinectedin Application Scope
Lurbinectedin is a drug used for the treatment of metastatic small-cell lung cancer (SCLC) in adult patients who have experienced disease progression during or after platinum-based chemotherapy. It exhibits significant antitumor activity by inhibiting the transcription process in tumor cells and modulating the tumor microenvironment.

Zepzelca Package Insert
Ingredients:
- Active ingredient: Lurbinectedin
- Excipients: Not explicitly stated (specific excipients should be referred to in the drug package insert).
Properties:
- White to off-white lyophilized powder, requiring reconstitution before injection.
- Administered via intravenous infusion, with a unique transcription inhibition mechanism.
Specification:
- Each vial contains 4 mg of Lurbinectedin (specific specifications may vary by manufacturer).
Packaging Specification:
- Typically supplied as single-dose vials, with each box containing 1 or more vials (specific packaging details should refer to the manufacturer’s information).
Storage:
- Store at 2–8°C under refrigeration, protected from light and freezing.
- Reconstituted solutions should be used within 24 hours.
Expiry Date:
- Usually 24 months (specific expiration date should be checked on the packaging).
Executive Standard:
- Complies with FDA and EMA Good Manufacturing Practice (GMP) standards.
Approval Number:
- FDA Approval Number: NDA 213702 (accelerated approval in 2020).
Date of Revision:
- Latest revision date: November 2022 (specific date should be confirmed in the drug package insert).
Manufacturer:
- Developed and manufactured by PharmaMar.
Guidelines For The Use Of Lurbinectedin
Dosage and Administration:
- Recommended dose: 3.2 mg/m² administered intravenously every 21 days, with an infusion duration of approximately 60 minutes.
- Must be administered under the supervision of a healthcare professional.
Zepzelca Side Effects
- Common adverse reactions: Leukopenia, lymphopenia, fatigue, anemia, nausea, decreased appetite, thrombocytopenia, etc.
- Serious adverse reactions may include myelosuppression and abnormal liver function.
Contraindications:
- Hypersensitivity to Lurbinectedin or any of its components.
- Use with caution in patients with severe hepatic impairment.
Precautions:
- Regular monitoring of blood counts and liver function is required during treatment.
- Pregnant and breastfeeding women should avoid use unless the potential benefits outweigh the risks.
Lurbinectedin Interactions
Drug Interactions:
- CYP3A4 inhibitors or inducers: May affect the metabolism of Lurbinectedin, requiring dose adjustments or avoidance of concomitant use.
- Myelosuppressive drugs: May increase the risk of myelosuppression, requiring close monitoring of blood counts.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from HongKong DengYue Medicine. It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.
Contact Us








Reviews
There are no reviews yet.