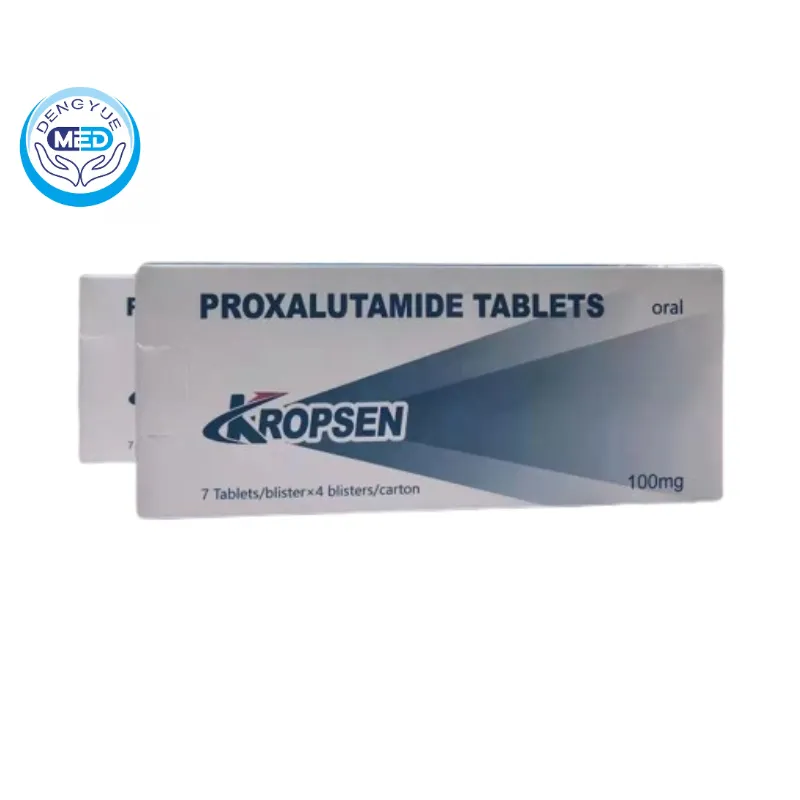
Kropsen (Proxalutamide) – mCRPC | HongKong DengYue Medicine
- Generic Name/Brand Name: Proxalutamide/Kropsen
- Indications: mCRPC
- Dosage Form: Tablets
- Specification: 100 mg x 28
Proxalutamide Application Scope
Proxalutamide (GT-0918) is a nonsteroidal antiandrogen and selective high-affinity silent antagonist of the androgen receptor under investigation for multiple therapeutic applications. The primary indications being studied include prostate cancer (particularly metastatic castration-resistant prostate cancer [mCRPC]) and coronavirus disease 2019 (COVID-19). Additionally, proxalutamide is being explored for the treatment of breast cancer and other androgen receptor-related conditions.
For COVID-19 treatment, proxalutamide has been evaluated for both hospitalized and non-hospitalized patients with mild to moderate disease, as well as severe or critically ill patients. For prostate cancer, the drug has advanced through Phase II clinical trials in mCRPC patients.
Proxalutamide Characteristics
-
Ingredients: Proxalutamide is the active pharmaceutical ingredient. The chemical name is 4-[4,4-dimethyl-3-[6-[3-(2-oxazolyl)propyl]-3-pyridinyl]-5-oxo-2-thioxo-1-imidazolidinyl]-3-fluoro-2-(trifluoromethyl)benzonitrile, with alternative designations including GT0918, GT-0918, and Pruxelutamide. Additional tablet components include pharmaceutical excipients required for formulation stability and bioavailability.
-
Properties: Proxalutamide is a nonsteroidal antiandrogen (NSAA) and a selective high-affinity silent antagonist of the androgen receptor (AR). It inhibits androgen-induced receptor activation, forms inactive complexes that prevent nuclear translocation, and induces AR downregulation. It is a small-molecule oral drug with the chemical formula C₂₄H₁₉F₄N₅O₂S and a molar mass of 517.50 g/mol. It has low water solubility (0.0379 mg/mL) and is predicted to have moderate lipophilicity (logP around 4.5-4.65).
-
Packaging Specification: 100mg x 7 x 4 tablets.
-
Storage: Proxalutamide tablets should be stored at room temperature (approximately 20-25°C). The drug product should be maintained in a secure, environmentally controlled, and monitored storage area with access limited to authorized personnel. The drug must be protected from moisture and light exposure during storage.
-
Expiry Date: The shelf-life of proxalutamide is 2 years when stored under appropriate conditions.
-
Executive Standard: Proxalutamide is developed under investigational drug standards and is classified as a Class 1.1 innovative drug for certain indications. The drug development follows International Council for Harmonization (ICH) standards including ICH E6 guidelines for good clinical practice.
-
Approval Number: Proxalutamide received an Emergency Use Authorization (EUA) from Paraguay’s Ministry of Public Health and Social Welfare (MSPBS) in July 2021 for the treatment of hospitalized COVID-19 patients. This represents the first overseas authorization for proxalutamide. The drug has also received FDA approval for Phase III clinical trials in the United States (IND: 154032).
-
Date of Revision: The clinical protocols for proxalutamide COVID-19 studies were released and amended between 2021 and 2022. The Phase III COVID-19 protocol (GT0918-US-3001) was finalized on July 8, 2021, with Amendment 1 released on June 11, 2021.
-
Manufacturer: Suzhou Kintor Pharmaceuticals, Inc. (also known as Kintor Pharmaceutical Limited, HKEX: 9939) is the primary developer and manufacturer of proxalutamide. Established in 2009, the company is headquartered in Suzhou, China, and specializes in the development of innovative androgen receptor antagonist therapies. Kintor Pharmaceutical received funding for proxalutamide development under China’s “13th Five-Year Plan” for major novel drug innovation.
Guidelines for the Use of Proxalutamide
-
Dosage and Administration:
-
Recommended Dose:
The recommended dose varies depending on the clinical indication:
-
COVID-19 (Mild to Moderate): 200 mg once daily (administered as 2 tablets of 100 mg each)
-
COVID-19 (Hospitalized/Severe): 200 mg once daily
-
Prostate Cancer (mCRPC): 100 mg, 200 mg, or 300 mg once daily, with 200 mg/day identified as the optimal dose for Phase III trials
-
Breast Cancer: Doses studied range from 100 mg to 500 mg daily, with 200-300 mg/day identified for further evaluation
-
-
Administration: Administer orally once daily at the same time each day (±2 hours), with or without food. Swallow tablets whole; do not crush or chew.
-
Missed Dose: If a dose of proxalutamide is missed, it should not be made up later. The patient should resume the normal dosing schedule on the following day. If vomiting occurs after taking a dose, no re-dosing should occur before the next scheduled dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Fatigue, nausea, diarrhea, headache, vomiting, abdominal pain, hormonal imbalances, gynecomastia, musculoskeletal pain, and neurological effects.
-
Serious Adverse Reactions: Liver function abnormalities (elevated enzymes), cardiovascular effects, severe hormonal disruptions, and potential for severe gastrointestinal issues. Rare but serious events may include anaphylaxis or severe fatigue leading to falls.
-
-
Contraindications: Hypersensitivity to proxalutamide or its components. Not recommended during pregnancy, breastfeeding, or in women of childbearing potential without effective contraception due to potential fetal harm. Avoid in patients with severe hepatic impairment.
-
Precautions:
-
Monitor liver function tests regularly due to risk of hepatotoxicity. Use caution in patients with cardiovascular disease, as antiandrogens may exacerbate risks. Monitor for signs of hormonal imbalance or gynecomastia. Not studied in pediatric populations or severe renal impairment. Discontinue if severe adverse reactions occur. Proxalutamide may affect fertility; discuss with patients.
-
Proxalutamide Interactions
-
Limited specific interaction data is available due to its investigational status. As an androgen receptor antagonist, it may interact with other hormonal therapies or AR modulators. Potential for drug-drug interactions via CYP enzymes (e.g., CYP3A4 inducers/inhibitors may affect levels, similar to related drugs like enzalutamide or apalutamide). Avoid concomitant use with strong CYP3A4 inducers (e.g., rifampin) or inhibitors (e.g., ketoconazole). May enhance effects of anticoagulants or increase exposure to substrates of certain transporters. Consult a healthcare provider for polypharmacy assessment.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.





Reviews
There are no reviews yet.