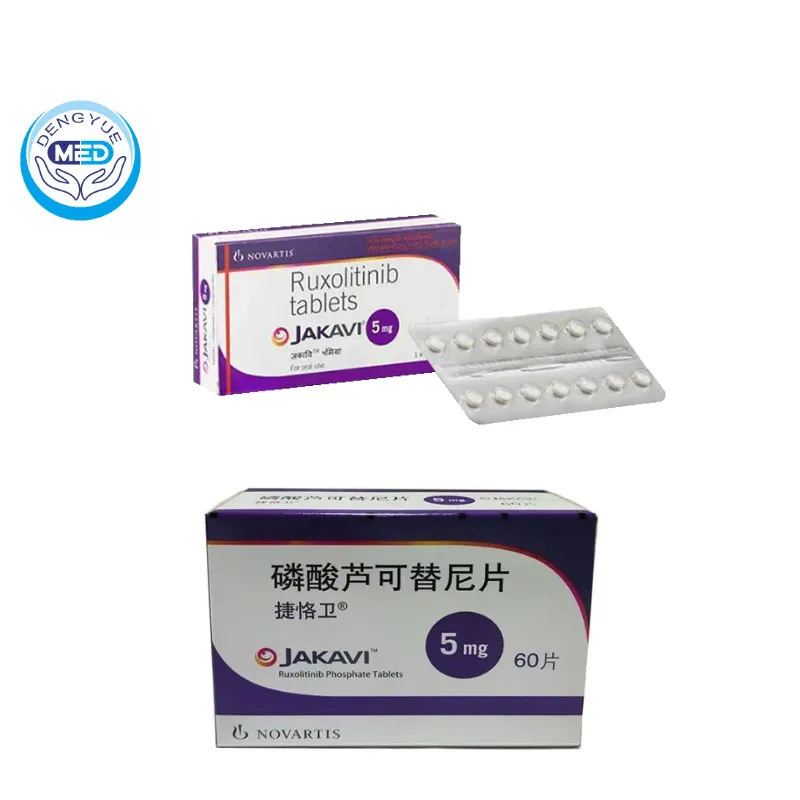
JAKAVI (Ruxolitinib) – MF/GVHD | HongKong DengYue Medicine
- Generic Name/Brand Name: Ruxolitinib/JAKAVI
- Indications: Myelofibrosis, Polycythemia Vera, Graft-versus-Host Disease
- Dosage Form: Film-coated Tablet
- Specification: 5 mg/15 mg/20 mg × 60 tablets
JAKAVI Application Scope
-
Treatment of myelofibrosis (MF) in adult patients: for disease‑related splenomegaly or symptoms in:
-
Primary (chronic idiopathic) myelofibrosis,
-
Post-polycythemia vera MF,
-
Post-essential thrombocythemia MF.
-
-
Polycythemia vera (PV) in adult patients who are resistant to or intolerant of hydroxyurea.
-
Graft-versus-host disease (GvHD):
-
Acute GvHD: in adults (and pediatric in some regions) with inadequate response to corticosteroids or other systemic therapies.
-
Chronic GvHD (in some approvals)—see clinical data (e.g., REACH3 trial)

jakavi ruxolitinib
-
JAKAVI Characteristics
-
Ingredients: Ruxolitinib
-
Properties: Film-coated tablet
-
Packaging Specification: Are available in multiple strengths (e.g., 5 mg, 15 mg, 20 mg)
-
Storage: Store below 30°C (86°F)
-
Expiry Date: As stated on the packaging
-
Executive Standard: Approved and regulated by local health authorities
-
Approval Number: Varies by country and region (e.g., EU/1/12/791/001-013)
-
Date of Revision: This is product-specific and should be checked on the latest package insert
-
Manufacturer: Novartis Pharma Stein AG, Switzerland, or other licensed facilities
Guidelines for the Use of JAKAVI
-
Dosage and Administration:
-
Recommended Dose:
The starting dose is individualized based on platelet count and the condition being treated.
-
Myelofibrosis: Starting dose typically ranges from 15 mg twice daily to 20 mg twice daily.
-
Polycythemia Vera: Starting dose is typically 10 mg twice daily.
-
GVHD: Starting dose is typically 10 mg twice daily.
-
-
Administration: Taken orally with or without food. Tablets should be swallowed whole with water.
-
Missed Dose: If a dose is missed, it should be taken as soon as it is remembered unless it is less than 6 hours until the next dose. Do not take a double dose to make up for a forgotten dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Thrombocytopenia (low platelet count), anemia (low red blood cell count).
-
Bruising, dizziness, headache.
-
Increased blood cholesterol and liver enzymes (ALT/AST).
-
Bacterial infections, herpes zoster (shingles), and urinary tract infections.
-
-
Serious Adverse Reactions:
-
Severe infections (e.g., tuberculosis, sepsis).
-
Progressive Multifocal Leukoencephalopathy (PML).
-
New non-melanoma skin cancers.
-
Major adverse cardiovascular events (MACE).
-
Severe hepatic toxicity.
-
-
-
Contraindications:
-
Hypersensitivity to ruxolitinib or any of the excipients.
-
Concomitant use with strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole) in patients with renal or hepatic impairment.
-
-
Precautions:
-
Complete Blood Counts (CBC): Must be monitored frequently as thrombocytopenia, anemia, and neutropenia are common. Dose adjustments or interruptions may be necessary.
-
Infections: Active serious infections should be resolved before starting treatment. Patients should be monitored for signs and symptoms of infection and treated promptly.
-
Lipids: Lipid parameters should be monitored approximately 8-12 weeks after initiation.
-
Hepatotoxicity: Liver function should be monitored periodically.
-
Non-Melanoma Skin Cancer: Periodic skin examinations are recommended.
-
Pregnancy & Lactation: Not recommended during pregnancy unless clearly necessary. Breastfeeding is not recommended during treatment.
-
JAKAVI Interactions
-
Strong CYP3A4 Inhibitors (e.g., ketoconazole, fluconazole, clarithromycin): Significantly increase ruxolitinib exposure. Concomitant use is not recommended, or a dose reduction of JAKAVI is required.
-
Immunosuppressants: May increase the risk of infection.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.








Reviews
There are no reviews yet.