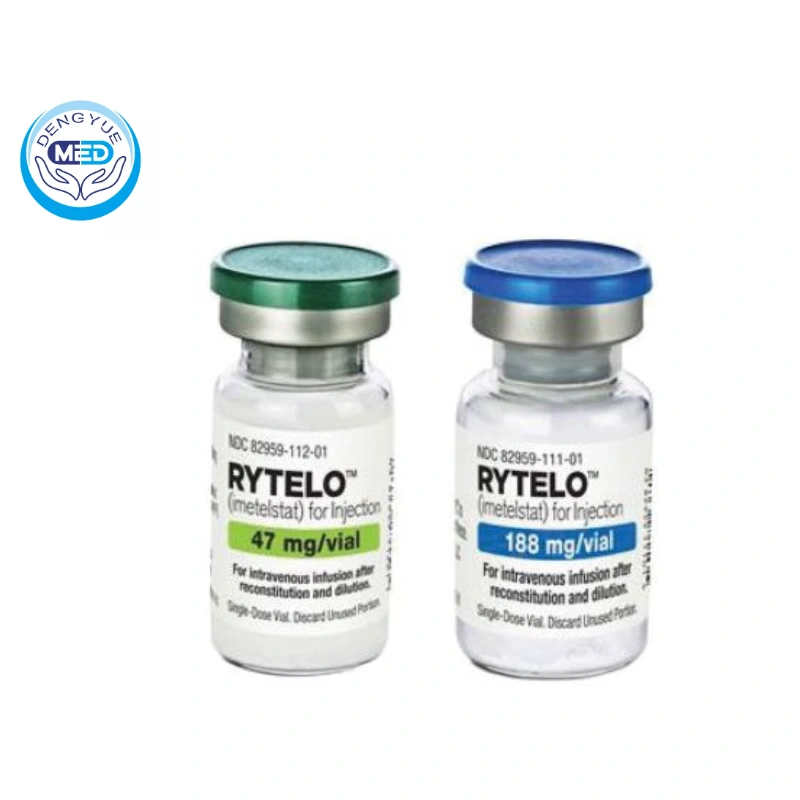
Imetelstat For Injection|Myelodysplastic Syndromes|HongKong DengYue Medicine
- Generic Name/Brand Name: Imetelstat / Rytelo
- Indications: Treatment of low- to intermediate-1 risk myelodysplastic syndromes with transfusion-dependent anemia.
- Dosage Form: Intravenous infusion.
- Specification: Available in 188 mg and 47 mg vials.
Imetelstat For Injection Application Scope
Imetelstat for Injection, marketed under the brand name Rytelo, is an oligonucleotide telomerase inhibitor approved for the treatment of certain myelodysplastic syndromes (MDS). Below is detailed information about this medication:
Imetelstat is indicated for adult patients with low- to intermediate-1 risk MDS who have transfusion-dependent anemia requiring four or more red blood cell (RBC) units over 8 weeks and have not responded to, have lost response to, or are ineligible for erythropoiesis-stimulating agents (ESAs).
Imetelstat For Injection Characteristics
-
Ingredients: Imetelstat sodium.
-
Properties: Oligonucleotide telomerase inhibitor.
-
Specification: Available in 188 mg and 47 mg strengths.
-
Dosage Form: Powder for solution for intravenous infusion.
-
Packaging Specification: Supplied in single-use vials.
-
Storage: Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton. Do not freeze.
-
Expiry Date: Refer to the packaging for the expiration date.
-
Executive Standard: Refer to the prescribing information for detailed standards.
-
Approval Number: Consult local regulatory authorities for the specific approval number.
-
Date of Revision: Refer to the latest prescribing information for the revision date.
-
Manufacturer: Geron Corporation.
Guidelines for Use of Imetelstat For Injection
-
Dosage and Administration: The recommended dosage is 7.1 mg/kg administered as an intravenous infusion over 2 hours every 4 weeks. Pretreatment medications, such as diphenhydramine and hydrocortisone, are recommended at least 30 minutes prior to dosing to reduce infusion-related reactions. Monitor patients for at least 1 hour after infusion completion.
-
Adverse Reactions: Common adverse reactions (≥10%) include decreased platelets, decreased white blood cells, decreased neutrophils, increased liver enzymes (aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase), fatigue, prolonged partial thromboplastin time, joint and muscle pain, infections (including COVID-19), and headache.
-
Contraindications: There are no listed contraindications. However, patients should be monitored closely due to the potential for severe adverse reactions.
-
Precautions: Imetelstat can cause severe myelosuppression, leading to neutropenia and thrombocytopenia. Liver function abnormalities have been observed; therefore, regular monitoring of blood counts and liver function tests is essential. Patients should avoid live vaccinations during treatment and be cautious of potential infections. Effective contraception is advised during treatment and for 1 week after the last dose due to potential harm to a developing fetus. Breastfeeding is not recommended during treatment and for 1 week after the last dose.
Imetelstat For Injection Interactions
-
Drug Interactions: Live vaccines should be avoided during treatment with imetelstat due to the risk of infections. Inform your healthcare provider about all medications and supplements you are taking to assess potential interactions.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.





Reviews
There are no reviews yet.