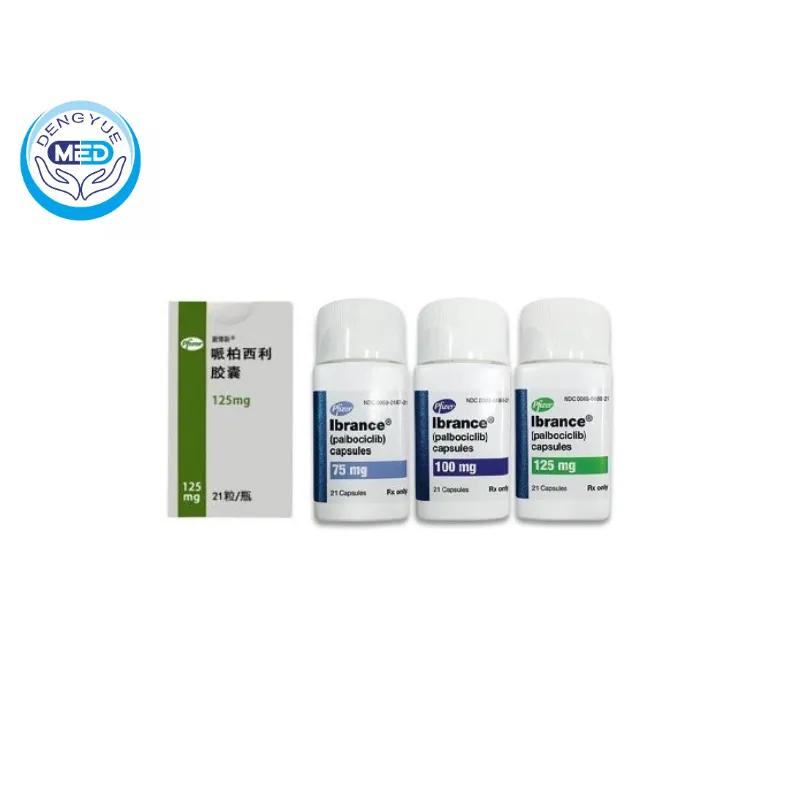
Ibrance (Palbociclib) | Breast Cancer | HongKong DengYue
- Generic Name/Brand Name: Palbociclib/Ibrance
- Indications: Breast cancer
- Dosage Form: Oral capsules or film-coated tablets
- Specification: 75 mg, 100 mg, and 125 mg x 21 capsules
Palbociclib Application Scope
-
Treatment of adult patients with hormone receptor (HR)-positive, HER2-negative advanced or metastatic breast cancer in combination with an aromatase inhibitor (initial therapy) or fulvestrant (after progression)
-
Additionally approved with inavolisib and fulvestrant for endocrine-resistant, PIK3CA-mutated advanced breast cancer

Palbociclib Characteristics
-
Ingredients:
-
Active: palbociclib
-
Other excipients: capsules contain gelatin, printing (“PBC 125” etc.), tablets are film-coated (detailed in EMA packaging info)
-
-
Properties: A selective CDK4/6 kinase inhibitor that blocks G1 to S-phase cell cycle progression in HR+ breast cancer cells
-
Packaging Specification:
-
Capsules: Bottles of 21 capsules (75, 100, or 125 mg) labeled “PBC xx”
-
Tablets: Blister packs of 21 or 63 film‑coated tablets (75, 100, 125 mg), PVC/Al blisters
-
-
Storage:
-
Store at 20–25 °C (68–77 °F); allowed variation 15–30 °C (59–86 °F)
-
Tablets should remain in original blister packaging to protect from moisture
-
-
Expiry Date: Unkonwn
-
Executive Standard:
-
U.S. FDA standards (NDA 207103, initial approval 2015, latest labeling 2025)
-
EMA marketing authorisation (e.g. PLGB 00057/1699), renewal in July 2021; text updated Jan 2025
-
-
Approval Number:
-
U.S.: NDA 207103 (approved April 4, 2019 for updated indications)
-
EU: EU/1/16/1147 series (for different strengths)
-
-
Date of Revision: Recent US label changes in April 2025; EU text revised January 2025
-
Manufacturer: Pfizer Inc. (U.S., Ireland, Germany manufacturing)
Guidelines for the Use of Palbociclib
-
Dosage and Administration:
-
Standard dosing: 125 mg once daily for 21 days, followed by 7 days off (28-day cycle); take with food
-
Can be taken with or without food for tablets; capsules require food
-
Dose adjustments for hepatic impairment: 75 mg daily in severe impairment (Child‑Pugh C); no adjustment in mild–moderate cases
-
Combination regimens:
-
With aromatase inhibitor (e.g., letrozole 2.5 mg daily)
-
With fulvestrant (500 mg on Days 1, 15, 29, then monthly)
-
With inavolisib + fulvestrant for PIK3CA-mutant disease
-
-
-
Adverse Reactions:
Common:
-
Neutropenia (most frequent), leukopenia, anemia, fatigue, nausea, diarrhea, infections, headache, thrombocytopenia, decreased appetite
-
Serious: Risk of febrile neutropenia, pulmonary embolism, fetal harm in pregnancy
-
-
Contraindications: Hypersensitivity to palbociclib or any excipient
-
Precautions:
-
Monitor CBC frequently due to neutropenia risk
-
Avoid in pregnancy; counsel on effective contraception
-
Caution in severe hepatic impairment; adjust dose accordingly
-
Interactions
- Drug Interactions:
-
CYP3A inhibitors (e.g., ketoconazole): may increase palbociclib exposure; avoid strong inhibitors
-
CYP3A inducers (e.g., rifampin): may reduce exposure; avoid
-
Sensitive CYP3A substrates: palbociclib may increase their levels—monitor or adjust dose
-
Grapefruit: avoid due to CYP3A inhibition risk
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- It is only for internal discussion among medical staff and does not serve as a basis for medication. For specific medication guidelines, please consult the attending physician.





Reviews
There are no reviews yet.