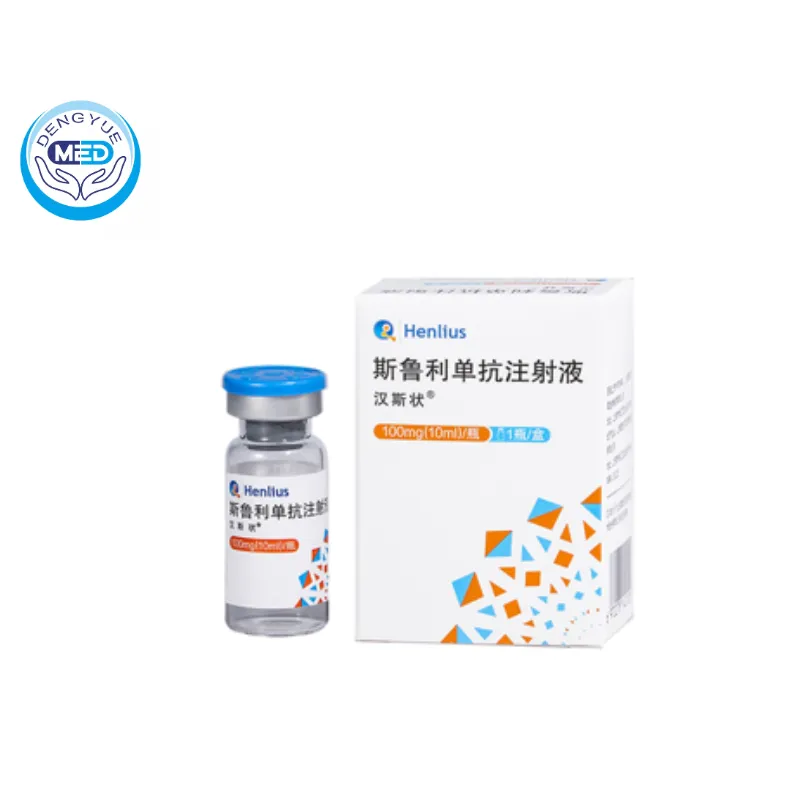
Hansizhuang (Serplulimab) – Solid Tumors | HongKong DengYue Medicine
- Generic Name/Brand Name: Serplulimab/Hansizhuang
- Indications: ES‑SCLC, NSCLC, ESCC, MSI‑H/dMMR solid tumors, Gastric cancer
- Dosage Form: Concentrate for solution for intravenous infusion
- Specification: 100 mg/10 ml × 1 vial
Serplulimab Application Scope
Serplulimab is indicated for the treatment of the following malignancies (as approved by the NMPA of China):
-
First-line treatment of Extensive-stage Small Cell Lung Cancer (ES-SCLC) in combination with carboplatin and etoposide.
-
First-line treatment of metastatic non-squamous Non-Small Cell Lung Cancer (NSCLC) in combination with pemetrexed and carboplatin.
-
First-line treatment of metastatic squamous Non-Small Cell Lung Cancer (NSCLC) in combination with paclitaxel and carboplatin.
-
Previously treated MSI-H (Microsatellite Instability-High) solid tumors as a single agent.
-
Previously treated Esophageal Squamous Cell Carcinoma (ESCC) as a single agent.

hansizhuang serplulimab
Serplulimab Characteristics
-
Ingredients: Serplulimab
-
Properties: A clear to slightly opalescent, colorless to slightly yellow liquid for intravenous infusion
-
Packaging Specification: 100 mg/10 mL per vial
-
Storage: Store at 2°C to 8°C (36°F to 46°F) in the original outer carton to protect from light
-
Expiry Date: 24 months
-
Executive Standard: The drug is manufactured and released in compliance with the approved specifications of the National Medical Products Administration (NMPA) of China
-
Approval Number: 国药准字 S20220013
-
Date of Revision: The latest version of the prescribing information should be consulted
-
Manufacturer: Shanghai Henlius Biotech, Inc.
Guidelines for the Use of Serplulimab
-
Dosage and Administration:
-
Recommended Dose:
-
For ES‑SCLC (first‑line, in clinical trials / EU regimen): 4.5 mg/kg IV every 3 weeks, combined with carboplatin (AUC=5, up to 750 mg) + etoposide (100 mg/m² on days 1–3) for induction; maintenance: serplulimab 4.5 mg/kg every 3 weeks until disease progression or unacceptable toxicity.
-
For MSI‑H / dMMR solid tumors (per earlier Chinese approval): some literature reports 3 mg/kg IV every 2 weeks until disease progression or intolerance.
-
For other indications (NSCLC, ESCC), dose/schedule may follow clinical guidelines—typically under the doctor’s decision.
-
-
Administration:
-
Intravenous infusion. For example, EU SmPC: infused once every 3 weeks; first infusion ~ 1 hour; if tolerated, later infusions may be shorter (~ 30 minutes).
-
Preparation: vial is diluted in 0.9% sodium chloride (normal saline), filtered through a 0.2–5 μm filter, then administered. After infusion, flush IV line with saline.
-
Stability once diluted: use immediately; if not, may keep up to 24 hours at 2–8°C (max 6 hours at ≤ 25°C).
-
-
Missed Dose: If a dose is missed, administer as soon as possible. Then, adjust the schedule to maintain the 3-week interval. Do not administer two doses simultaneously to make up for a missed one.
-
-
Adverse Reactions:
-
Common Adverse Reactions:
-
Elevated liver enzymes (AST/ALT), elevated bilirubin
-
Proteinuria, decreased albumin levels, decreased white blood cell count / neutrophil count / lymphocyte count / platelets
-
Anemia, fatigue, decreased appetite, nausea, abdominal pain, diarrhea/constipation, rash/skin reactions
-
Thyroid dysfunction (hypothyroidism / hyperthyroidism)
-
Other: potentially general immune‑related events typical of PD-1 blockade
-
-
Serious Adverse Reactions:
-
Immune-mediated pneumonitis
-
Immune-mediated colitis
-
Immune-mediated hepatitis
-
Immune-mediated endocrinopathies (e.g., thyroid disorders, adrenal insufficiency, type 1 diabetes)
-
Immune-mediated skin adverse reactions
-
Immune-mediated nephritis
-
Infusion-related reactions
-
-
-
Contraindications: Hypersensitivity to serplulimab or any of its excipients.
-
Precautions:
-
Immune-Mediated Adverse Reactions (irAEs): Can be severe or fatal. Monitor patients closely for signs and symptoms of irAEs. Most irAEs improve with corticosteroid treatment and, if necessary, therapy interruption or discontinuation.
-
Infusion-Related Reactions: Interrupt or slow the rate of infusion for mild to moderate reactions. Permanently discontinue for severe or life-threatening reactions.
-
Complications of Allogeneic HSCT: Fatal and other serious complications can occur after allogeneic hematopoietic stem cell transplantation (HSCT).
-
Use in Special Populations: Use during pregnancy may cause fetal harm. Advise women of the potential risk. It is unknown whether serplulimab is present in human milk. The safety and effectiveness in pediatric patients have not been established.
-
Serplulimab Interactions
-
There is limited publicly available data on drug–drug interactions. As with other immunotherapies, concomitant use of systemic immunosuppressants (corticosteroids, other immunosuppressive agents) may reduce efficacy.
-
Use of live vaccines or other immune‑modulating therapies during treatment is generally not recommended (common with PD-1 inhibitors), though specific instructions may depend on local clinical guidelines.
-
Always check with treating physician/pharmacist for potential interactions (especially with chemotherapy agents, supportive care drugs, etc.).
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.







Reviews
There are no reviews yet.