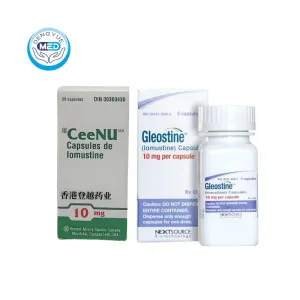
Gleostine (Lomustine) – Glioma | HongKong DengYue Medicine
- Generic Name/Brand Name: Lomustine / Gleostine®
- Indications: Glioma
- Dosage Form: Oral capsules
- Specification: 10 mg × 10 tablets/bottle
Lomustine Application Scope
Gleostine® is primarily indicated for the treatment of glioma, including glioblastoma multiforme (GBM) and other malignant brain tumors. It is used for patients with recurrent or difficult-to-treat gliomas, either as monotherapy or as part of combination chemotherapy, particularly when other treatments have limited efficacy.
Lomustine Characteristics
-
Ingredients: Lomustine
-
Properties: Oral alkylating agent, crosses blood-brain barrier
-
Packaging Specification: 10 mg × 10 tablets/box
-
Storage: Store in a cool, dry place, away from light
-
Expiry Date: See packaging
-
Executive Standard: Drug Registration Regulations
-
Approval Number: NMPA H20140001
-
Date of Revision: June 2022
-
Manufacturer: NextSource Biotechnology
Guidelines for the Use of Gleostine
-
Dosage and Administration:
-
Missed Dose: If a dose is missed, administer as soon as possible. Resume monthly dosing from the date of the most recently administered dose.
-
-
Adverse Reactions:
-
Common Adverse Reactions (≥5%):
-
nausea, vomiting, decreased blood counts, fatigue
-
-
Serious Adverse Reactions:
-
hypersensitivity reactions, severe myelosuppression.
-
-
-
Contraindications: Patients with known severe hypersensitivity to it
-
or excipients
-
Precautions:
-
Monitor blood counts and liver function during treatment
-
Advise patients to inform healthcare provider if pregnant, planning pregnancy, or breastfeeding
-
Lomustine Interactions
- Consult healthcare provider for potential drug interactions
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.






Reviews
There are no reviews yet.