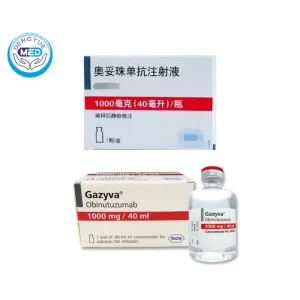
Gazyva (Obinutuzumab) – Follicular Lymphoma | HongKong DengYue Medicine
- Generic Name/Brand Name: Obinutuzumab/Gazyva
- Indications: Chronic Lymphocytic Leukemia (CLL), Follicular Lymphoma (FL)
- Dosage Form: Injection
- Specification: 1000mg 40ml x 1 vial
Obinutuzumab Application Scope
-
Chronic Lymphocytic Leukemia (CLL): In combination with chlorambucil for patients with previously untreated CLL.
-
Follicular Lymphoma (FL):
-
In combination with bendamustine followed by obinutuzumab monotherapy for patients who relapsed after or are refractory to a rituximab-containing regimen.
-
In combination with chemotherapy followed by obinutuzumab monotherapy in patients achieving at least a partial remission, for previously untreated stage II bulky, III, or IV FL.
-
Obinutuzumab Characteristics
-
Ingredients: Each vial contains 1,000 mg of obinutuzumab in 40 mL of solution, providing a concentration of 25 mg/mL.
-
Properties: It is a humanized anti-CD20 monoclonal antibody of the IgG1 isotype.
-
Packaging Specification: Single-dose vial containing 1,000 mg/40 mL.
-
Storage: Store in a refrigerator at 2°C to 8°C (36°F to 46°F). Protect from light. Do not freeze. Do not shake.
-
Expiry Date: Refer to the expiration date stamped on the carton.
-
Executive Standard: Complies with the U.S. Food and Drug Administration (FDA) standards.
-
Approval Number: FDA Approval No. 125486s029.
-
Date of Revision: February 2022.
-
Manufacturer: Genentech, a member of the Roche Group.
Guidelines for the Use of Obinutuzumab
-
Dosage and Administration:
-
Recommended Dose:
-
CLL: Administer 100 mg intravenously on Day 1, 900 mg on Day 2, and 1,000 mg on Days 8 and 15 of Cycle 1. For Cycles 2 through 6, administer 1,000 mg on Day 1 of each cycle.
-
FL:
-
Relapsed/Refractory FL: Combine with bendamustine for six 28-day cycles, followed by monotherapy.
-
Previously Untreated FL: Combine with chemotherapy (e.g., CHOP or CVP) followed by monotherapy in patients achieving at least a partial remission.
-
-
-
Administration: Administer as an intravenous infusion. Premedication may be required to reduce the risk of infusion-related reactions.
-
Missed Dose: If a dose is missed, administer as soon as possible. Do not administer two doses on the same day.
-
-
Adverse Reactions:
-
Common Adverse Reactions: Infusion-related reactions, fatigue, neutropenia, cough, upper respiratory tract infections, musculoskeletal pain.
-
Serious Adverse Reactions: Progressive multifocal leukoencephalopathy (PML), hepatitis B virus reactivation, severe infusion-related reactions, infections.
-
-
Contraindications:
-
Hypersensitivity to it or any of its components.
-
Active hepatitis B infection.
-
Severe active infections.
-
-
Precautions:
-
Monitor for infusion-related reactions during and after administration.
-
Screen for hepatitis B virus infection prior to treatment.
-
Use with caution in patients with a history of cardiovascular disease.
-
Obinutuzumab Interactions
-
Immunosuppressive Agents: Concurrent use may increase the risk of infections and neutropenia.
-
Vaccines: Avoid live vaccines during treatment.
-
Other Antineoplastic Agents: Use with caution due to potential additive immunosuppressive effects.
Note:
- If there is a new packaging for the drug, the new packaging shall prevail. The above information is sourced from DengYue Medicine.
- This content is for reference only. Prescription drugs must be used under a doctor’s guidance and purchased from authorized sources.









Reviews
There are no reviews yet.